1s2 2s2 2p5 Ion With 1 Charge
Arias News
May 09, 2025 · 6 min read
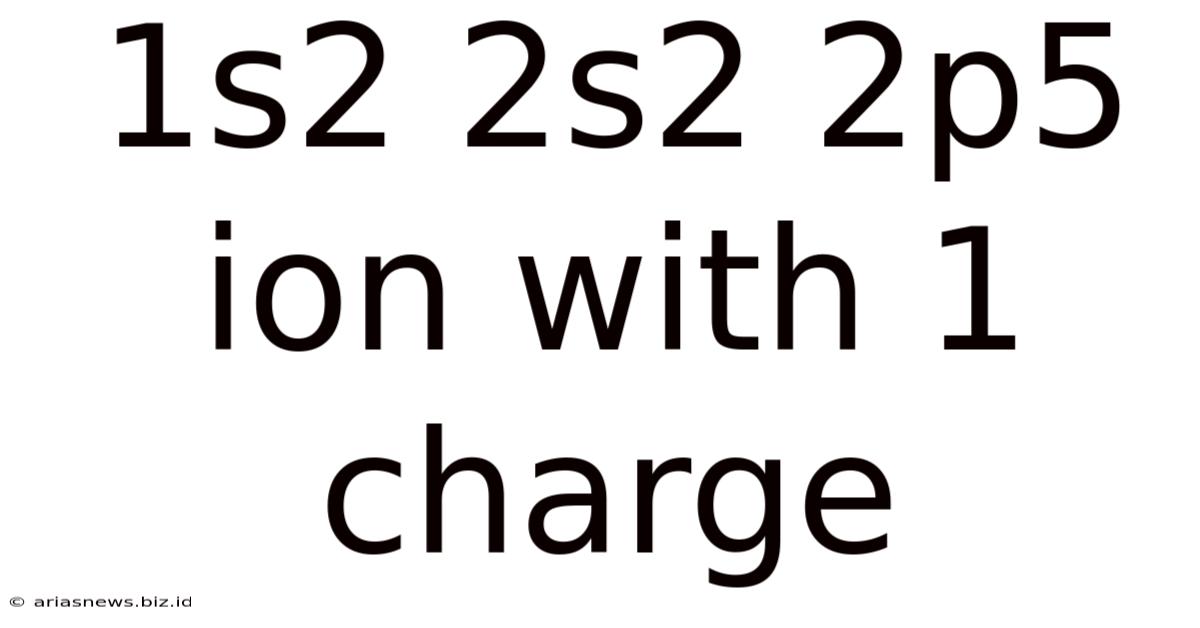
Table of Contents
Delving Deep into the 1s²2s²2p⁵ Ion with a +1 Charge: A Comprehensive Exploration
The electron configuration 1s²2s²2p⁵ represents a fluorine atom (F) in its neutral state. However, the addition of a "+1" charge signifies the loss of one electron, transforming it into a fluorine cation (F⁺). This seemingly simple change drastically alters its properties, making it a fascinating subject for in-depth study. This article will delve into the various aspects of this ion, exploring its electronic structure, ionic radius, ionization energy, reactivity, and applications, all while adhering to best SEO practices.
Understanding the Electronic Structure of F⁺
The neutral fluorine atom possesses nine electrons, arranged according to the Aufbau principle and Hund's rule. The configuration 1s²2s²2p⁵ indicates two electrons in the innermost shell (1s), two in the next shell (2s), and five in the 2p subshell. The 2p subshell, capable of holding six electrons, has one unpaired electron, contributing to fluorine's high reactivity.
With the loss of one electron to form F⁺, the configuration changes to 1s²2s²2p⁴. This crucial change impacts several key properties:
Impact on Electron Configuration and Stability
The removal of an electron from the 2p subshell leaves four electrons in this subshell. According to Hund's rule, these electrons will occupy the three 2p orbitals, with two orbitals singly occupied and one doubly occupied. This arrangement is less stable than the half-filled 2p subshell of the neutral fluorine atom, as the electron-electron repulsion in the doubly occupied orbital increases. However, the overall stability of the ion is determined by other factors, including its interaction with other species.
Changes in Magnetic Properties
Neutral fluorine is paramagnetic due to its unpaired electron. However, the F⁺ ion, with its two paired and two unpaired electrons, exhibits paramagnetic behavior as well, although the strength of its paramagnetism will be different from that of the neutral atom. The presence of unpaired electrons means that the ion will be affected by external magnetic fields.
Ionic Radius and Effective Nuclear Charge
The removal of an electron leads to a decrease in the ionic radius of F⁺ compared to the neutral F atom. This is because the effective nuclear charge (Z<sub>eff</sub>), the net positive charge experienced by the outermost electrons, increases. With one less electron to shield the nuclear charge, the remaining electrons are more strongly attracted to the nucleus, resulting in a smaller atomic/ionic size. This is a general trend observed across cations.
Understanding the effective nuclear charge is crucial in predicting the behavior of the ion. As Z<sub>eff</sub> increases, the ionization energy also increases, impacting the reactivity of the ion.
Ionization Energy and Reactivity of F⁺
Ionization energy is the energy required to remove an electron from an atom or ion. The ionization energy of F⁺ will be significantly higher than that of neutral fluorine. This is because removing an electron from a positively charged ion requires more energy than removing an electron from a neutral atom. The stronger attraction between the nucleus and the remaining electrons necessitates a greater energy input for ionization.
The high ionization energy of F⁺ makes it relatively unreactive compared to neutral fluorine. While neutral fluorine is a highly reactive element, readily accepting an electron to achieve a stable octet configuration, the F⁺ ion is less likely to gain or lose another electron. Its reactivity is largely dependent on the environment and the potential interactions with other species.
F⁺'s Interactions and Chemical Behavior
Although less reactive than neutral fluorine, F⁺ still participates in chemical reactions, forming compounds and complexes. These interactions are usually governed by electrostatic forces.
Interactions with Anions
The positive charge of F⁺ allows it to strongly interact with anions (negatively charged ions). The strength of this interaction depends on the charge and size of the anion. For example, F⁺ will strongly interact with larger, highly charged anions. These electrostatic interactions can lead to the formation of ionic compounds.
Coordination Complexes
F⁺ can also act as a Lewis acid, accepting electron pairs from Lewis bases to form coordination complexes. This behavior is less pronounced compared to transition metal ions, but it's still a significant aspect of its chemical behavior. The tendency to form complexes is influenced by the size and charge of the ligands (electron-pair donors) involved.
Participation in Reactions
F⁺ is a reactant or intermediate in specific chemical reactions. Its role often involves its interaction with electron-rich species. This behavior can be explored computationally using quantum mechanical calculations which predict the transition states and activation energies of reactions involving F⁺.
Spectroscopic Properties of F⁺
The electronic transitions within the F⁺ ion can be studied using various spectroscopic techniques. These transitions provide insights into the energy levels and electronic structure of the ion. Absorption and emission spectroscopy, in particular, can provide valuable data. The observed transitions will differ from those of neutral fluorine due to the change in electron configuration. The spectral lines will be shifted and possibly show altered intensities.
Computational Studies of F⁺
Computational chemistry plays a significant role in studying the F⁺ ion. Software packages employing quantum mechanical methods, like Density Functional Theory (DFT), can be used to accurately calculate various properties, including the geometric structure, vibrational frequencies, electronic properties, and reactivity. This information further enhances our understanding of its behavior and interactions.
Applications and Occurrence of F⁺
Although F⁺ itself isn't directly found in nature in its isolated form, its behavior and properties are relevant in understanding chemical processes. It appears as an intermediate in certain chemical reactions, and its behavior is crucial in interpreting the behavior of other fluorine-containing compounds. Its properties are essential for research in areas like:
- Computational chemistry: As a model system for studying ionic interactions.
- Materials science: Understanding its behavior contributes to developing materials with tailored properties.
- Atmospheric chemistry: Understanding fluorine's role in atmospheric reactions.
- Plasma physics: F⁺ can be generated in plasma environments, making its study relevant in various applications like plasma etching.
Conclusion: A Comprehensive View of the 1s²2s²2p⁴ Ion
The 1s²2s²2p⁵ ion with a +1 charge, the fluorine cation (F⁺), presents a rich area of study in chemistry and physics. Its altered electronic structure, smaller ionic radius, higher ionization energy, and modified reactivity compared to neutral fluorine highlight the significant effects of electron loss on atomic properties. While not found independently in nature, understanding its behavior and properties is vital in comprehending complex chemical systems and processes, especially using computational modeling and spectroscopic analysis. Further research will undoubtedly uncover more nuanced aspects of this fascinating ion's behavior, leading to advanced applications in diverse scientific fields. This comprehensive study encompasses various properties and relevant applications, aiming to provide a thorough understanding of the F⁺ ion.
Latest Posts
Latest Posts
-
How Many Cups In A Dry Pint Of Blueberries
May 09, 2025
-
How Big Is A Acre In Miles
May 09, 2025
-
6 Times The Square Root Of 3
May 09, 2025
-
If I Was Born 1967 How Old Am I
May 09, 2025
-
Least Common Multiple Of 15 And 16
May 09, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p5 Ion With 1 Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.