1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Arias News
Mar 18, 2025 · 6 min read
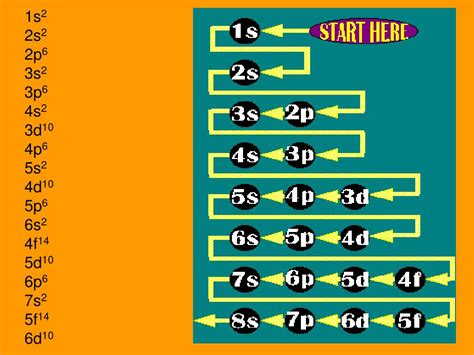
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²: Unveiling the Secrets of Electron Configuration
The seemingly cryptic sequence "1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²" represents much more than just numbers and letters. This is, in fact, the electron configuration of an element, a fundamental concept in chemistry that unveils the arrangement of electrons within an atom's orbitals. Understanding electron configuration provides crucial insights into an element's chemical properties, reactivity, and place within the periodic table. This detailed exploration will dissect the meaning of this specific configuration, delve into the principles governing electron arrangement, and explore the properties of the element it represents.
Understanding Electron Configuration: The Building Blocks of Atoms
Before we decipher "1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²," let's establish the fundamental principles. Electron configuration describes the distribution of electrons among the various energy levels and sublevels within an atom. This distribution follows specific rules:
The Aufbau Principle: Filling Orbitals in Order of Energy
The Aufbau principle states that electrons first fill the lowest energy levels available. Imagine it like building a house – you wouldn't start with the roof before laying the foundation. The energy levels are represented by the principal quantum numbers (n = 1, 2, 3, etc.), with higher numbers indicating greater energy.
The Pauli Exclusion Principle: One Electron Per Orbital with Specific Spin
The Pauli exclusion principle dictates that each orbital can hold a maximum of two electrons, provided they have opposite spins. Each electron possesses an intrinsic property called spin, which can be either "up" (↑) or "down" (↓). Therefore, an orbital can only accommodate one electron with spin "up" and one with spin "down".
Hund's Rule: Maximizing Unpaired Electrons
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes the total spin of the atom, creating a more stable configuration.
Deciphering 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²: A Step-by-Step Breakdown
Now, let's break down the electron configuration "1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²":
-
1s²: This indicates that the first energy level (n=1) contains one subshell, the 's' subshell, with two electrons. The 's' subshell can only hold a maximum of two electrons.
-
2s²: The second energy level (n=2) also has an 's' subshell with two electrons.
-
2p⁶: The second energy level also contains a 'p' subshell, which can hold up to six electrons (two electrons per orbital, and three orbitals in the 'p' subshell). This 'p' subshell is fully occupied.
-
3s²: The third energy level (n=3) starts with its 's' subshell, again containing two electrons.
-
3p⁶: The 'p' subshell of the third energy level is also fully occupied with six electrons.
-
4s²: The fourth energy level (n=4) begins with its 's' subshell filled with two electrons. Note that the 4s subshell fills before the 3d subshell due to the slightly lower energy level of 4s.
-
3d¹⁰: The 'd' subshell of the third energy level, capable of holding ten electrons (five orbitals x two electrons each), is completely filled.
-
4p²: Finally, the 'p' subshell of the fourth energy level contains two electrons. This subshell can accommodate up to six electrons.
Identifying the Element: The Significance of the Electron Configuration
The complete electron configuration, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p², identifies the element Lead (Pb). Lead's atomic number is 82, which precisely corresponds to the total number of electrons represented in this configuration (2 + 2 + 6 + 2 + 6 + 2 + 10 + 2 = 32).
Properties of Lead (Pb): A Consequence of its Electron Configuration
Lead's unique properties are directly influenced by its electron configuration:
Chemical Reactivity: Relatively Unreactive
Lead is a relatively unreactive metal, though it does react slowly with oxygen and acids. This relative inertness is partially due to its complete 3d and 6s subshells. These filled shells create a stable electron configuration, reducing its tendency to readily lose or gain electrons.
Density and Metallic Character: A Heavy Metal
Lead's electron configuration contributes to its high density and characteristic metallic properties. The presence of many electrons in various shells leads to strong metallic bonding, influencing its malleability and conductivity.
Toxicity: Environmental Concerns
Lead's toxicity is a well-known concern. The electronic structure does not directly explain this toxicity, but the chemical reactivity of lead ions in biological systems plays a crucial role. Its ability to mimic essential metal ions in enzymatic processes contributes to its toxicity.
Beyond the Basics: Further Exploration of Electron Configuration
While the electron configuration of lead provides a foundational understanding of its properties, several other advanced concepts are pertinent:
Orbital Diagrams: Visualizing Electron Arrangement
Orbital diagrams provide a visual representation of electron configuration, showing the specific occupancy of each orbital within a subshell. For example, the 4p² configuration of lead would show two unpaired electrons within the three 4p orbitals.
Ionization Energy and Electron Affinity: Understanding Reactivity
Ionization energy refers to the energy required to remove an electron from an atom. Lead's electron configuration suggests that removing electrons from its filled subshells would require significantly more energy compared to removing electrons from partially filled subshells.
Electron affinity is the energy change when an electron is added to a neutral atom. Lead's relatively low electron affinity is consistent with its overall low reactivity.
Exceptions to the Rules: Orbital Stability
Although the Aufbau principle provides a general framework, there are exceptions where the actual electron configuration deviates from the predicted order. This often occurs due to the relatively similar energy levels of certain orbitals, leading to increased stability in configurations with half-filled or fully filled subshells.
Applications of Lead: Industrial and Historical Uses
Lead's properties, governed by its electron configuration, have led to a wide array of applications throughout history. Its malleability, density, and relative inertness have contributed to its use in:
- Lead-acid batteries: Lead's reactivity with sulfuric acid, albeit slow, makes it a key component in rechargeable batteries.
- Radiation shielding: Its high density effectively absorbs radiation.
- Ammunition: Its malleability and density make it suitable for bullets and shot.
- Traditional plumbing: Although now largely phased out due to toxicity concerns, lead was historically used in pipes.
Conclusion: The Power of Electron Configuration in Understanding Matter
The seemingly simple sequence "1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²" holds the key to understanding the fundamental properties of lead. Electron configuration, governed by the Aufbau principle, the Pauli exclusion principle, and Hund's rule, provides a powerful framework for predicting and understanding the behavior of elements. By analyzing this configuration, we gain insights into lead's reactivity, density, and ultimately, its various applications and environmental impacts. This detailed examination illustrates the profound connection between an element's microscopic structure and its macroscopic properties, underscoring the central role of electron configuration in the study of chemistry.
Latest Posts
Latest Posts
-
How Can You Tell If A Shark Likes You
Mar 18, 2025
-
Is It An Hour Or A Hour
Mar 18, 2025
-
How To Say Passion Fruit In Spanish
Mar 18, 2025
-
How Many Hours Are In 180 Minutes
Mar 18, 2025
-
How Many Acers Are In A Mile
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
