Btu To Evaporate 1 Pound Of Water
Arias News
Apr 07, 2025 · 6 min read
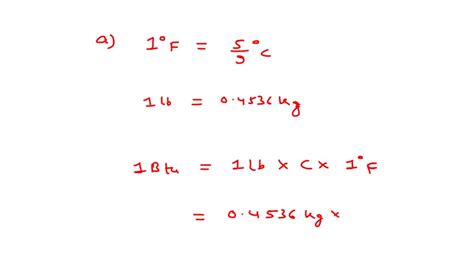
Table of Contents
BTU to Evaporate 1 Pound of Water: A Deep Dive into Latent Heat
Understanding the energy required to evaporate water is crucial in various fields, from HVAC design to industrial processes. This article delves deep into the concept of BTU (British Thermal Unit) and its relationship to evaporating one pound of water, exploring the underlying principles of latent heat and its practical implications. We'll also examine factors influencing this energy requirement and offer practical examples to solidify your understanding.
Understanding BTU and Latent Heat
Before diving into the specifics, let's establish a firm grasp of the fundamental concepts.
What is a BTU?
A BTU, or British Thermal Unit, is a unit of energy. It's defined as the amount of heat required to raise the temperature of one pound of liquid water by one degree Fahrenheit at sea level. While seemingly simple, it's a cornerstone in understanding energy transfer and consumption in numerous applications.
Latent Heat of Vaporization
This is where the magic happens. Latent heat is the energy absorbed or released during a phase transition, without a change in temperature. For water, the latent heat of vaporization refers to the energy required to change its state from liquid to vapor (steam). This energy doesn't increase the water's temperature; instead, it breaks the bonds holding the water molecules together in the liquid state, allowing them to escape as vapor.
This is significantly different from sensible heat, which is the energy that does change the temperature of a substance. You need both sensible and latent heat to evaporate water. You first heat the water (sensible heat) to its boiling point (212°F or 100°C at standard atmospheric pressure), and then supply additional energy (latent heat) to turn it into steam.
BTU to Evaporate 1 Pound of Water: The Calculation
The commonly accepted value for the latent heat of vaporization of water at standard atmospheric pressure (14.7 psi) is approximately 970 BTU per pound. This means that it takes approximately 970 BTUs of energy to completely evaporate one pound of water that's already at its boiling point.
However, the real-world scenario is more nuanced. Let's break down the process step-by-step, considering the energy requirements at each stage:
-
Heating the water to its boiling point: This depends on the initial temperature of the water. If the water starts at room temperature (around 70°F), you'll need additional energy to heat it to 212°F. The calculation for sensible heat is:
- Q (sensible heat) = m * c * ΔT
Where:
- Q = heat energy (BTU)
- m = mass of water (pounds)
- c = specific heat capacity of water (approximately 1 BTU/lb°F)
- ΔT = change in temperature (°F)
So, for 1 pound of water heated from 70°F to 212°F:
- Q = 1 lb * 1 BTU/lb°F * (212°F - 70°F) = 142 BTU
-
Evaporating the water: Once the water reaches its boiling point, you need the latent heat of vaporization to convert it to steam:
- Q (latent heat) = 970 BTU/lb * 1 lb = 970 BTU
-
Total Energy Required: To find the total energy required, simply add the sensible heat and latent heat:
- Total Q = 142 BTU + 970 BTU = 1112 BTU
Therefore, to evaporate one pound of water starting at 70°F, you'll need approximately 1112 BTU. Remember, this is an approximation, and the exact value may vary slightly based on pressure and other factors.
Factors Affecting the BTU Requirement
Several factors can influence the actual BTU requirement to evaporate one pound of water:
-
Initial Water Temperature: As shown in the example, a lower initial temperature requires more sensible heat to reach the boiling point, thus increasing the total energy needed.
-
Pressure: Atmospheric pressure affects the boiling point of water. Higher pressure increases the boiling point, requiring more energy to reach it, while lower pressure decreases the boiling point, requiring less.
-
Humidity: High humidity slows down the evaporation process because the air is already saturated with water vapor. This can indirectly affect the energy requirement by prolonging the evaporation time and potentially leading to heat losses.
-
Air Movement: Increased air movement improves heat transfer, accelerating the evaporation process and potentially reducing the overall energy consumption per unit time. However, the total energy remains the same.
-
Impurities in Water: The presence of dissolved salts or other impurities can slightly alter the latent heat of vaporization. However, this effect is usually negligible in most practical scenarios.
-
Heat Transfer Efficiency: The method used to heat and evaporate the water influences efficiency. Different methods have varying degrees of heat loss, impacting the actual BTU requirement.
Practical Applications and Examples
Understanding BTU and latent heat is critical in numerous applications:
-
HVAC Systems: Air conditioning and refrigeration systems rely on the evaporation of refrigerants to cool the air. Calculating the BTU requirements is essential for designing efficient and effective systems.
-
Industrial Processes: Many industrial processes, such as steam generation and distillation, require accurate estimations of energy consumption for evaporation. This is crucial for optimizing production and minimizing energy costs.
-
Boiler Design: Boilers are designed to generate steam, and understanding the BTU requirement per pound of water is fundamental to sizing the boiler correctly and ensuring efficient operation.
-
Weather Forecasting: Latent heat plays a significant role in weather patterns and atmospheric processes. Accurate prediction of evaporation rates is critical for weather forecasting models.
Beyond the Basics: Advanced Considerations
While the basic calculation provides a good starting point, several advanced considerations refine the accuracy:
-
Thermodynamic Properties of Water: For highly precise calculations, utilizing steam tables and thermodynamic property software is necessary. These tables provide values of enthalpy and entropy at various temperatures and pressures, giving more accurate values for latent heat and sensible heat calculations.
-
Heat Transfer Mechanisms: Conduction, convection, and radiation all contribute to heat transfer during evaporation. A comprehensive analysis should account for all three mechanisms to accurately determine the energy requirement.
-
Energy Loss: Heat loss to the surroundings due to conduction, convection, and radiation significantly impacts the actual BTU requirement. Minimizing heat loss through proper insulation and design is crucial for improving efficiency.
Conclusion: Mastering the BTU to Evaporate Water
The seemingly simple question of how many BTUs it takes to evaporate one pound of water leads us into the fascinating world of latent heat and its practical significance. While the approximate value of 970 BTU/lb for latent heat provides a useful starting point, a truly comprehensive understanding requires considering several factors including initial water temperature, pressure, humidity, air movement, and heat transfer efficiency. By mastering these concepts, engineers, designers, and scientists can optimize energy usage in various applications, contributing to greater efficiency and sustainability. Remember that precise calculations require utilizing steam tables and sophisticated software to account for all thermodynamic properties and heat transfer mechanisms. This deep dive serves as a foundation for more advanced explorations in the world of thermodynamics and heat transfer.
Latest Posts
Latest Posts
-
How To Say Good Morning In Hawaiian
Apr 08, 2025
-
How Old Are You If Born In 1991
Apr 08, 2025
-
What Is 72 Cm In Waist Size
Apr 08, 2025
-
Is 1 8 Greater Than 1 2
Apr 08, 2025
-
Are Lays Sour Cream And Onion Gluten Free
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Btu To Evaporate 1 Pound Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.