Calculate The Mass Of 2.50 104 Molecules Of Nitrogen Gas
Arias News
May 10, 2025 · 5 min read
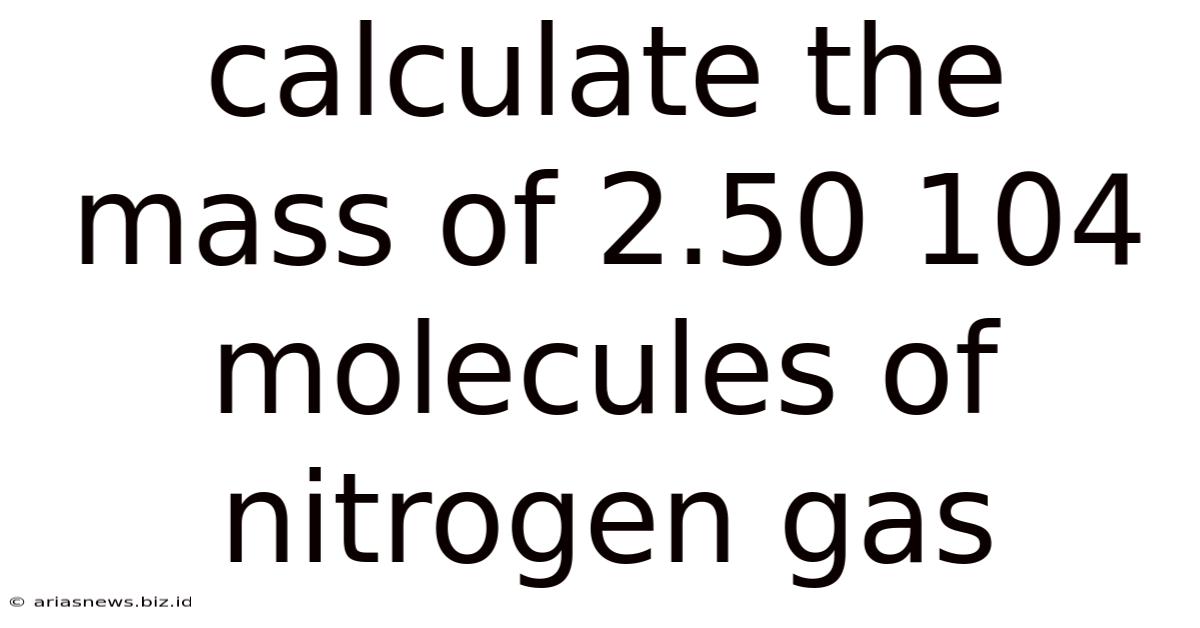
Table of Contents
Calculating the Mass of 2.50 x 10<sup>4</sup> Molecules of Nitrogen Gas: A Step-by-Step Guide
Determining the mass of a given number of molecules requires a fundamental understanding of chemistry, specifically molar mass, Avogadro's number, and the relationship between mass, moles, and the number of molecules. This article will guide you through the calculation of the mass of 2.50 x 10<sup>4</sup> molecules of nitrogen gas (N<sub>2</sub>), providing a detailed explanation of each step involved. We'll also explore related concepts and potential applications of this type of calculation.
Understanding the Fundamentals
Before we delve into the calculation, let's review some crucial concepts:
1. Molar Mass
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). One mole is defined as 6.022 x 10<sup>23</sup> elementary entities (atoms, molecules, ions, etc.), a number known as Avogadro's number (N<sub>A</sub>). For nitrogen gas (N<sub>2</sub>), the molar mass is calculated by adding the atomic masses of two nitrogen atoms:
- Atomic mass of Nitrogen (N) ≈ 14.01 g/mol
- Molar mass of Nitrogen gas (N<sub>2</sub>) = 2 * 14.01 g/mol = 28.02 g/mol
2. Avogadro's Number
Avogadro's number (N<sub>A</sub> = 6.022 x 10<sup>23</sup> mol<sup>-1</sup>) is a fundamental constant in chemistry. It represents the number of constituent particles (atoms, molecules, ions, etc.) present in one mole of any substance. This constant acts as a bridge between the macroscopic world (grams) and the microscopic world (number of molecules).
3. Relationship between Mass, Moles, and Number of Molecules
The key relationships we'll use are:
- Moles (n) = Mass (m) / Molar mass (M)
- Number of molecules (N) = Moles (n) * Avogadro's number (N<sub>A</sub>)
These equations allow us to convert between mass, moles, and the number of molecules.
Calculating the Mass
Now, let's calculate the mass of 2.50 x 10<sup>4</sup> molecules of nitrogen gas:
Step 1: Calculate the number of moles.
We know the number of molecules (N = 2.50 x 10<sup>4</sup>) and Avogadro's number (N<sub>A</sub> = 6.022 x 10<sup>23</sup> mol<sup>-1</sup>). We can rearrange the equation Number of molecules (N) = Moles (n) * Avogadro's number (N<sub>A</sub>) to solve for moles:
n = N / N<sub>A</sub>
n = (2.50 x 10<sup>4</sup> molecules) / (6.022 x 10<sup>23</sup> molecules/mol)
n ≈ 4.15 x 10<sup>-20</sup> mol
Step 2: Calculate the mass.
We now know the number of moles (n ≈ 4.15 x 10<sup>-20</sup> mol) and the molar mass of nitrogen gas (M = 28.02 g/mol). Using the equation Moles (n) = Mass (m) / Molar mass (M), we can solve for mass:
m = n * M
m = (4.15 x 10<sup>-20</sup> mol) * (28.02 g/mol)
m ≈ 1.16 x 10<sup>-18</sup> g
Therefore, the mass of 2.50 x 10<sup>4</sup> molecules of nitrogen gas is approximately 1.16 x 10<sup>-18</sup> grams.
Significance and Applications
This seemingly small mass highlights the incredibly large number of molecules present in even tiny amounts of a substance. The ability to convert between the number of molecules and mass is crucial in various fields:
-
Chemistry: Stoichiometric calculations, determining reaction yields, and understanding chemical reactions at a molecular level.
-
Material Science: Analyzing the composition and properties of materials at the nanoscale.
-
Environmental Science: Measuring pollutant concentrations and assessing environmental impact.
-
Biochemistry and Molecular Biology: Understanding biological processes at the molecular level, studying protein interactions, and analyzing DNA and RNA concentrations.
-
Pharmacology: Determining drug dosages and understanding drug efficacy.
Further Exploration
This calculation can be extended to other molecules and compounds by simply substituting the appropriate molar mass. Remember to always use the correct units and pay attention to significant figures in your calculations. Understanding the relationships between mass, moles, and the number of molecules is a foundational concept in chemistry with broad applications in various scientific disciplines.
Error Analysis and Precision
It's important to acknowledge potential sources of error in this calculation. The primary source of uncertainty stems from the use of an approximate value for Avogadro's number. While the value is incredibly precise, any slight deviation can affect the final result. Furthermore, the atomic masses used are average atomic weights, representing the weighted average of isotopes found in naturally occurring nitrogen. The actual mass of individual nitrogen molecules might vary slightly depending on isotopic composition.
Therefore, the final answer (1.16 x 10<sup>-18</sup> g) should be considered an approximation. The number of significant figures reflects the precision of the input values and the inherent uncertainties in the calculations.
Conclusion
This article provided a comprehensive guide to calculating the mass of 2.50 x 10<sup>4</sup> molecules of nitrogen gas. By understanding the concepts of molar mass, Avogadro's number, and their interrelationships, you can successfully perform similar calculations for any substance. This fundamental skill is essential for anyone studying chemistry or related scientific fields. Remember to always consider potential sources of error and use appropriate significant figures in your calculations. Mastering this skill opens doors to a deeper understanding of the quantitative aspects of the molecular world.
Latest Posts
Latest Posts
-
Saquen Una Hoja De Papel In English
May 10, 2025
-
1 2 Ounce Is Equal To How Many Teaspoons
May 10, 2025
-
What Are The Solutions To The Inequality Mc003 1 Jpg
May 10, 2025
-
1 2 Cup Of Sour Cream In Oz
May 10, 2025
-
How Long Does 2 Miles Take To Walk
May 10, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Mass Of 2.50 104 Molecules Of Nitrogen Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.