Does Mercury Have More Protons And Electrons Than Tin
Arias News
Mar 17, 2025 · 5 min read
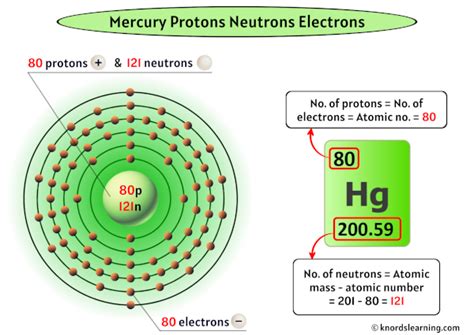
Table of Contents
Does Mercury Have More Protons and Electrons Than Tin? Unveiling the Atomic Secrets
Understanding the fundamental building blocks of matter—atoms—is crucial to answering questions about the properties and behavior of elements like mercury and tin. This article delves deep into the atomic structure of these two elements, comparing their number of protons and electrons to definitively answer the question: Does mercury have more protons and electrons than tin? We will explore the concepts of atomic number, atomic mass, isotopes, and their implications for understanding the differences between these metals.
Understanding Atomic Structure: Protons, Electrons, and Neutrons
Before we compare mercury and tin, let's refresh our understanding of atomic structure. An atom consists of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels. In a neutral atom, the number of electrons equals the number of protons.
- Neutrons: Neutrally charged particles residing in the nucleus. They contribute to the atom's mass but not its charge.
The atomic number is a unique identifier for each element on the periodic table. It corresponds to the number of protons in the nucleus. The atomic mass is the total mass of protons and neutrons in the nucleus, expressed in atomic mass units (amu). Isotopes are atoms of the same element with the same number of protons but a different number of neutrons, hence varying atomic masses.
Mercury (Hg): A Look at its Atomic Properties
Mercury, a heavy, silvery-white liquid metal at room temperature, is fascinating for its unique properties. Let's examine its atomic structure:
- Atomic Number: 80. This means a mercury atom has 80 protons in its nucleus.
- Number of Electrons: In a neutral mercury atom, the number of electrons equals the number of protons, so it also has 80 electrons.
- Atomic Mass: The atomic mass of mercury is approximately 200.59 amu. This is an average value because mercury exists as several stable isotopes, each with a different number of neutrons. The most abundant isotopes are Mercury-196, Mercury-198, Mercury-200, and Mercury-202. This variation in neutron number results in slightly different atomic masses for individual mercury atoms.
Tin (Sn): Exploring its Atomic Composition
Tin, a silvery-white metal known for its malleability and ductility, also holds a significant place in the periodic table. Let's explore its atomic characteristics:
- Atomic Number: 50. A tin atom possesses 50 protons in its nucleus.
- Number of Electrons: Similar to mercury, a neutral tin atom has 50 electrons orbiting its nucleus.
- Atomic Mass: The atomic mass of tin is approximately 118.71 amu. Like mercury, tin exhibits isotopic variation, leading to this average atomic mass. The most abundant isotopes are Tin-116, Tin-118, Tin-119, Tin-120, and Tin-124.
Comparing Mercury and Tin: Protons and Electrons
Now, we can directly compare the number of protons and electrons in mercury and tin atoms:
| Element | Atomic Number (Protons) | Number of Electrons (in neutral atom) |
|---|---|---|
| Mercury (Hg) | 80 | 80 |
| Tin (Sn) | 50 | 50 |
The comparison clearly demonstrates that mercury has significantly more protons and electrons than tin. Mercury's atomic number (80) is considerably higher than tin's (50), directly reflecting the greater number of protons and, consequently, electrons in a neutral mercury atom.
Implications of the Difference in Protons and Electrons
The difference in the number of protons and electrons between mercury and tin significantly impacts their chemical and physical properties. These differences manifest in various ways:
- Chemical Reactivity: The number of electrons in the outermost shell (valence electrons) dictates an element's reactivity. Mercury and tin have different numbers of valence electrons, leading to variations in their chemical behavior and the types of compounds they form.
- Melting and Boiling Points: The strength of metallic bonds, influenced by the number of electrons and the arrangement of atoms, affects the melting and boiling points of metals. Mercury's significantly lower melting point compared to tin is a result of weaker metallic bonds.
- Density: The mass of the atom and the atomic arrangement influence density. Mercury, with its higher atomic mass and specific atomic structure, has a higher density than tin.
- Electrical Conductivity: The availability of free electrons determines a metal's electrical conductivity. The differences in electron configurations affect the conductivity properties of mercury and tin.
Beyond Protons and Electrons: Isotopes and Atomic Mass
While the number of protons defines the element, isotopes introduce variations in atomic mass. Understanding isotopes is crucial for a complete picture of mercury and tin:
-
Mercury Isotopes: Several stable isotopes of mercury exist in nature. These variations in neutron numbers don't affect the chemical properties but do affect the mass and density of individual mercury atoms. The average atomic mass of mercury reflects the abundance of each isotope.
-
Tin Isotopes: Tin exhibits even greater isotopic variation than mercury, with ten stable isotopes. This abundance of isotopes contributes to the average atomic mass of tin, impacting its overall properties and applications.
The presence of isotopes contributes to the slightly different average atomic masses observed for both mercury and tin. These average masses are weighted averages reflecting the relative abundance of each isotope in nature.
Conclusion: A Clear Difference in Atomic Composition
In conclusion, the answer to the question "Does mercury have more protons and electrons than tin?" is a resounding yes. Mercury, with an atomic number of 80, possesses significantly more protons and electrons (in a neutral atom) than tin, with an atomic number of 50. This fundamental difference in atomic structure directly impacts their chemical reactivity, physical properties, and various applications. Understanding the concepts of atomic number, atomic mass, and isotopes provides a comprehensive understanding of the differences between these two fascinating metallic elements. The variations in isotopic abundance further contribute to the unique properties and behavior of both mercury and tin.
Latest Posts
Latest Posts
-
How Do You Spell 80 In Words
Mar 17, 2025
-
How Long Would It Take To Walk 8 Miles
Mar 17, 2025
-
How Many Cans In A 2 Liter
Mar 17, 2025
-
How Many Kisses In A 32 Oz Jar
Mar 17, 2025
-
How Many Cups In 4 Oz Of Chocolate Chips
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Mercury Have More Protons And Electrons Than Tin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
