How Does The Shape Of The Molecule Affect Its Polarity
Arias News
Mar 22, 2025 · 5 min read
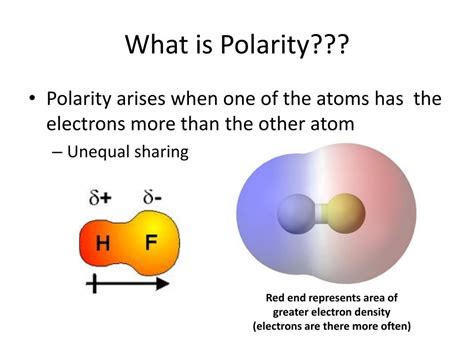
Table of Contents
How Does the Shape of a Molecule Affect its Polarity?
Molecular polarity, a fundamental concept in chemistry, significantly impacts a molecule's physical and chemical properties. Understanding how a molecule's shape influences its polarity is crucial for predicting its behavior in various contexts, from its solubility in different solvents to its reactivity in chemical reactions. This article delves into the intricate relationship between molecular geometry and polarity, exploring various factors and providing illustrative examples.
The Basics: Polarity and Electronegativity
Before diving into the impact of molecular shape, let's refresh our understanding of polarity and electronegativity. Polarity refers to the distribution of electrical charge within a molecule. A polar molecule possesses a positive and a negative end, creating a dipole moment. In contrast, a nonpolar molecule has an even distribution of charge.
Electronegativity, denoted by the Greek letter χ (chi), is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Elements with higher electronegativity values strongly attract electrons, while those with lower values attract electrons less strongly. The difference in electronegativity between atoms within a molecule determines the bond polarity.
A large electronegativity difference between bonded atoms results in a polar covalent bond, where electrons are unequally shared, leading to partial positive (δ+) and partial negative (δ-) charges on the atoms. A small electronegativity difference (or none) leads to a nonpolar covalent bond, where electrons are shared relatively equally.
Molecular Geometry: The Key Player
While electronegativity differences dictate the polarity of individual bonds, the overall polarity of a molecule depends critically on its three-dimensional shape or molecular geometry. This is because the individual bond dipoles can either reinforce or cancel each other out, depending on the spatial arrangement of the atoms.
Several theories predict molecular geometry, including Valence Shell Electron Pair Repulsion (VSEPR) theory. VSEPR theory postulates that electron pairs (both bonding and lone pairs) around a central atom repel each other and arrange themselves to minimize this repulsion, resulting in specific molecular geometries. These geometries directly influence the cancellation or addition of individual bond dipoles.
How Shape Affects Polarity: Case Studies
Let's examine several examples to illustrate how molecular shape influences polarity:
1. Carbon Dioxide (CO₂): A Linear, Nonpolar Molecule
Carbon dioxide has a linear geometry (O=C=O). Although the C=O bonds are polar (oxygen is significantly more electronegative than carbon), the two bond dipoles are equal in magnitude and point in opposite directions. They perfectly cancel each other out, resulting in a nonpolar CO₂ molecule. The symmetrical arrangement is crucial here.
2. Water (H₂O): A Bent, Polar Molecule
Water exhibits a bent molecular geometry due to the presence of two lone pairs on the oxygen atom. The O-H bonds are polar (oxygen is more electronegative than hydrogen), and the bond dipoles do not cancel each other out. Instead, they combine vectorially to create a net dipole moment, making water a polar molecule. The asymmetrical arrangement is key to its polarity.
3. Methane (CH₄): A Tetrahedral, Nonpolar Molecule
Methane possesses a tetrahedral geometry with four C-H bonds. While the C-H bonds exhibit slight polarity (carbon is slightly more electronegative than hydrogen), this difference is small. Moreover, the symmetrical tetrahedral arrangement ensures that the four bond dipoles cancel each other out completely, rendering methane a nonpolar molecule.
4. Ammonia (NH₃): A Trigonal Pyramidal, Polar Molecule
Ammonia features a trigonal pyramidal geometry due to the presence of a lone pair of electrons on the nitrogen atom. The N-H bonds are polar (nitrogen is more electronegative than hydrogen), and the bond dipoles, combined with the effect of the lone pair, do not cancel out. This results in a net dipole moment and makes ammonia a polar molecule. The asymmetrical arrangement and the influence of the lone pair contribute to the overall polarity.
5. Carbon Tetrachloride (CCl₄): A Tetrahedral, Nonpolar Molecule
Similar to methane, carbon tetrachloride has a tetrahedral geometry. However, the C-Cl bonds are significantly more polar than the C-H bonds in methane because of the higher electronegativity of chlorine compared to hydrogen. Despite the individual bond polarities, the symmetrical tetrahedral arrangement ensures complete cancellation of bond dipoles, leading to a nonpolar molecule.
The Role of Lone Pairs
Lone pairs of electrons significantly influence molecular shape and, consequently, polarity. Lone pairs occupy space around the central atom and exert repulsive forces on bonding pairs, affecting the bond angles and overall molecular geometry. As seen in the examples of water and ammonia, the presence of lone pairs often leads to asymmetrical geometries and, hence, polar molecules. The lone pairs contribute to the overall dipole moment by creating a region of higher electron density.
Implications of Molecular Polarity
The polarity of a molecule profoundly influences its properties and behavior:
-
Solubility: Polar molecules tend to dissolve in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents (like oil). This is based on the principle of "like dissolves like".
-
Boiling and Melting Points: Polar molecules generally have higher boiling and melting points than nonpolar molecules of similar size due to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding in the case of molecules with O-H, N-H, or F-H bonds).
-
Reactivity: Polar molecules often participate in different types of chemical reactions compared to nonpolar molecules. Their partial charges can facilitate interactions with other polar molecules or ions.
-
Spectroscopic Properties: Molecular polarity influences the absorption and emission of electromagnetic radiation, affecting techniques like infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy.
Conclusion
The relationship between molecular shape and polarity is complex but crucial for understanding chemical behavior. The spatial arrangement of atoms and the presence of lone pairs dictate whether individual bond dipoles cancel out or combine to create a net dipole moment. Predicting molecular polarity requires considering both the electronegativity differences between atoms and the molecule's three-dimensional geometry, as elucidated by theories like VSEPR. Understanding this relationship provides valuable insights into various chemical and physical properties and is essential for advancing our knowledge in diverse scientific fields. Further research into more complex molecules and advanced computational techniques continues to enhance our understanding of this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
How Much Does A Can Of Coke Weigh
Mar 23, 2025
-
Which Term Most Clearly Describes A Medium
Mar 23, 2025
-
How Much Protein Is 8 Oz Of Chicken
Mar 23, 2025
-
How Many Oz Is A Pound Of Pasta
Mar 23, 2025
-
How Many Cups In 5 Pounds Of Rice
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Does The Shape Of The Molecule Affect Its Polarity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
