How Many Cm Are In A Gram
Arias News
Mar 18, 2025 · 5 min read
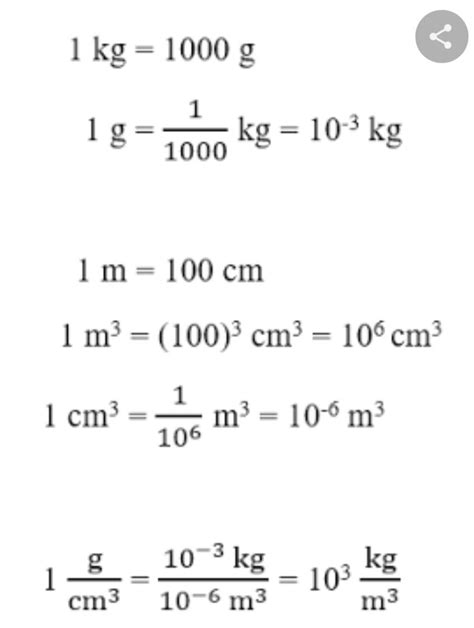
Table of Contents
How Many Centimeters Are in a Gram? Understanding Units of Measurement
The question "How many centimeters are in a gram?" reveals a fundamental misunderstanding of units of measurement. Centimeters (cm) measure length or distance, while grams (g) measure mass or weight. They are fundamentally different quantities and cannot be directly converted. It's like asking how many apples are in an orange – they're simply incomparable units. This article will delve into the distinction between these units, explore related concepts like volume and density, and offer practical examples to clarify the confusion.
Understanding Centimeters and Grams: A Crucial Distinction
Before we can address the core misconception, it's crucial to understand what each unit represents:
-
Centimeter (cm): A unit of length in the metric system. One centimeter is equal to one-hundredth of a meter (1 cm = 0.01 m). It's a common unit for measuring smaller distances, like the length of an object or the height of a person.
-
Gram (g): A unit of mass in the metric system. Mass represents the amount of matter in an object. While often used interchangeably with weight in everyday language, mass and weight are distinct concepts. Weight is the force exerted on an object due to gravity, while mass is an intrinsic property of the object. One gram is a relatively small unit of mass; 1000 grams equal one kilogram (1000 g = 1 kg).
The key takeaway here is that centimeters measure spatial extent, while grams measure the quantity of matter. They are fundamentally different dimensions and therefore cannot be converted into each other without additional information.
Introducing Volume and Density: The Missing Link
To establish a relationship between length (cm) and mass (g), we need to introduce two more concepts: volume and density.
-
Volume: This refers to the amount of three-dimensional space occupied by an object or substance. Volume is often measured in cubic centimeters (cm³) or liters (L). A cubic centimeter represents a cube with sides of 1 cm each.
-
Density: Density is the mass per unit volume of a substance. It tells us how much mass is packed into a given space. Density is usually expressed in grams per cubic centimeter (g/cm³). For instance, the density of water is approximately 1 g/cm³, meaning that one cubic centimeter of water has a mass of approximately one gram.
Density provides the bridge we need to connect mass (grams) and volume (which can be related to length in centimeters). If we know the density of a substance and its volume, we can calculate its mass. Conversely, if we know the mass and density, we can calculate its volume.
Calculating Mass from Volume and Density
Let's illustrate this with an example. Suppose we have a cube of aluminum with sides of 2 cm each. The volume of this cube is:
Volume = length x width x height = 2 cm x 2 cm x 2 cm = 8 cm³
The density of aluminum is approximately 2.7 g/cm³. To find the mass of the aluminum cube, we use the following formula:
Mass = Density x Volume = 2.7 g/cm³ x 8 cm³ = 21.6 g
Therefore, the aluminum cube with a volume of 8 cm³ (derived from its length measurements in cm) has a mass of 21.6 grams. Notice how we've indirectly connected centimeters (through volume) to grams.
Calculating Volume from Mass and Density
The reverse calculation is also possible. Let's say we have 54 grams of gold, and the density of gold is approximately 19.3 g/cm³. To find the volume of this gold:
Volume = Mass / Density = 54 g / 19.3 g/cm³ ≈ 2.8 cm³
This means that 54 grams of gold occupy a volume of approximately 2.8 cubic centimeters. Again, we've linked grams to a volume which, in principle, can be described using length dimensions in centimeters, though determining the precise shape with only volume would require additional information.
Real-World Applications and Examples
Understanding the relationship between mass, volume, density, and length is crucial in many real-world applications:
-
Engineering: Engineers use density calculations to determine the mass of materials needed for construction projects, ensuring structural integrity and stability.
-
Chemistry: Chemists use density to determine the concentration of solutions and to identify unknown substances.
-
Physics: Physicists utilize density in a variety of calculations, including fluid mechanics and thermodynamics.
-
Everyday Life: Even in everyday life, a basic understanding of these concepts helps with understanding cooking recipes (measuring ingredients by weight or volume), choosing appropriate containers for storage (ensuring they can hold a certain volume and weight), and so on.
Addressing Common Misconceptions
It's crucial to dispel common misunderstandings surrounding mass, volume, and density:
-
Mass vs. Weight: Remember, mass is the amount of matter, while weight is the force exerted due to gravity. Weight changes depending on gravitational pull (you'd weigh less on the moon), but mass remains constant.
-
Volume and Shape: Volume is independent of the shape of the object. Two objects with different shapes can have the same volume.
Conclusion
The initial question, "How many centimeters are in a gram?" is inherently flawed. Centimeters and grams measure different physical quantities: length and mass, respectively. To connect these units, we must consider volume and density. Density provides the crucial link, allowing us to calculate mass from volume (and length) or volume from mass. Understanding these relationships is fundamental in various scientific and engineering fields, as well as in many aspects of everyday life. By grasping the distinct concepts of mass, volume, and density, and their interrelationships, we can navigate the world of measurements with greater accuracy and understanding.
Latest Posts
Latest Posts
-
What Is A 12 Out Of 14
Mar 18, 2025
-
How Long Does It Take To Walk 14 Miles
Mar 18, 2025
-
How Long Is A 100 Mile Drive
Mar 18, 2025
-
How Many Cups In A Jar Of Peanut Butter
Mar 18, 2025
-
How Old Is Someone Born In 1971
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Cm Are In A Gram . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
