How Many Grams Are In 238 Moles Of Arsenic
Arias News
Mar 25, 2025 · 4 min read
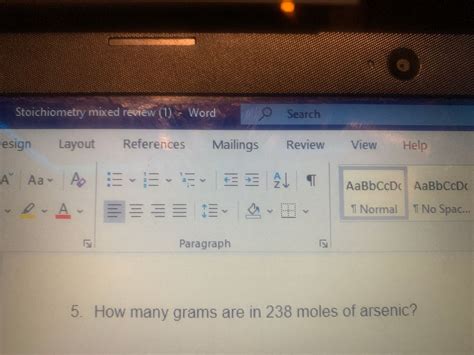
Table of Contents
How Many Grams are in 238 Moles of Arsenic? A Comprehensive Guide
Determining the mass of a given number of moles of a substance is a fundamental concept in chemistry. This article will delve into the process of calculating the grams in 238 moles of arsenic, exploring the underlying principles, providing a step-by-step solution, and discussing related concepts to enhance your understanding of stoichiometry.
Understanding Moles and Molar Mass
Before we tackle the calculation, let's clarify the core concepts involved:
-
Mole (mol): The mole is the International System of Units (SI) base unit for the amount of substance. One mole contains approximately 6.022 x 10²³ (Avogadro's number) entities, whether these are atoms, molecules, ions, or other specified particles. It's a crucial link between the microscopic world of atoms and molecules and the macroscopic world of grams and kilograms we experience daily.
-
Molar Mass (g/mol): The molar mass of an element or compound represents the mass of one mole of that substance in grams. It's essentially the atomic weight (for elements) or molecular weight (for compounds) expressed in grams per mole. You can find molar mass values on the periodic table or calculated from the atomic weights of constituent elements.
Calculating the Grams in 238 Moles of Arsenic
Arsenic (As) is a metalloid element with atomic number 33. To determine the mass of 238 moles of arsenic, we need its molar mass. Looking at a periodic table, we find the atomic weight of arsenic is approximately 74.92 g/mol.
Step 1: Identify the Known Values
- Moles of arsenic (n) = 238 mol
- Molar mass of arsenic (M) = 74.92 g/mol
Step 2: Apply the Formula
The relationship between moles (n), mass (m), and molar mass (M) is given by the formula:
m = n x M
Where:
- m = mass in grams
- n = number of moles
- M = molar mass in g/mol
Step 3: Perform the Calculation
Substitute the known values into the formula:
m = 238 mol x 74.92 g/mol
m = 17832.56 g
Therefore, there are approximately 17,832.56 grams in 238 moles of arsenic.
Significance and Applications
The ability to convert between moles and grams is crucial in numerous chemical applications, including:
-
Stoichiometric Calculations: Many chemical reactions are expressed in terms of moles. Converting moles to grams allows us to determine the actual mass of reactants or products involved in a reaction. This is vital for accurate experimental design and analysis.
-
Quantitative Analysis: Techniques like titration and gravimetric analysis rely on precise mass measurements. Understanding the relationship between moles and grams is essential for interpreting the results of these analytical procedures.
-
Material Science and Engineering: In material science and engineering, the precise control of the amounts of different elements in alloys and other materials is critical to achieve desired properties. Molar mass conversions are instrumental in ensuring the correct proportions are used.
-
Pharmaceutical Sciences: Accurate dosage calculations in pharmaceuticals depend heavily on converting between moles and grams to ensure the correct amount of active ingredients is present in medications.
Beyond the Basics: Dealing with Impurities and Uncertainties
The calculation above assumes pure arsenic. In real-world scenarios, samples often contain impurities. If you're working with an arsenic sample that's not 100% pure, you need to account for the percentage purity. For example, if your sample is 95% pure arsenic, you would adjust the calculation as follows:
Mass of pure arsenic = 17832.56 g x 0.95 = 16940.93 g
This highlights the importance of considering sample purity in any quantitative chemical analysis.
Furthermore, the molar mass value of 74.92 g/mol is an average atomic weight, considering the natural isotopic abundance of arsenic. There's a small degree of uncertainty associated with this value, which propagates through the calculation. For high-precision work, you should consult more precise molar mass values and consider error propagation techniques.
Expanding the Concept: Working with Compounds
The principles discussed here apply equally to compounds. For example, to calculate the mass of 238 moles of arsenic trioxide (As₂O₃), you would first determine the molar mass of As₂O₃ by summing the atomic weights of its constituent elements:
Molar mass of As₂O₃ = (2 x 74.92 g/mol) + (3 x 16.00 g/mol) = 197.84 g/mol
Then, you would use the same formula (m = n x M) to calculate the mass:
m = 238 mol x 197.84 g/mol = 47101.12 g
Conclusion
Calculating the mass of 238 moles of arsenic, or any substance for that matter, involves a straightforward application of the formula m = n x M. However, a deeper understanding of the underlying concepts of moles and molar mass, along with considerations for sample purity and uncertainty, is essential for accurate and meaningful results in chemical calculations and real-world applications. This knowledge forms a fundamental cornerstone of many scientific disciplines. Remember to always double-check your units and ensure that you are using consistent units throughout your calculations. Understanding these principles empowers you to confidently navigate the world of stoichiometry and related chemical computations.
Latest Posts
Latest Posts
-
How Many 2x6 Are In A Bundle
Mar 28, 2025
-
Which Shape Has 1 Vertex And 1 Circular Face
Mar 28, 2025
-
Multiplication Chart That Goes Up To 100
Mar 28, 2025
-
How Many Empty Cans Make A Pound
Mar 28, 2025
-
Open In A Wall To Let In Air Or Light
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Are In 238 Moles Of Arsenic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
