How Many Grams Are In 60 Ml
Arias News
Mar 17, 2025 · 5 min read
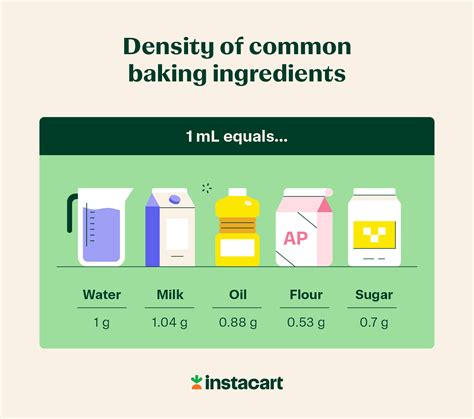
Table of Contents
How Many Grams Are in 60 ml? Understanding the Relationship Between Volume and Mass
Determining how many grams are in 60 ml isn't a straightforward answer. It depends entirely on the density of the substance being measured. Density is the mass per unit volume of a substance, typically expressed in grams per milliliter (g/ml) or grams per cubic centimeter (g/cm³). Since 1 ml is equivalent to 1 cm³, these units are interchangeable in most contexts.
This article will explore the relationship between volume (ml) and mass (grams), delve into the concept of density, and provide methods for calculating the mass of a substance given its volume and density. We'll also examine common substances and their densities to illustrate this crucial concept.
The Importance of Density in Conversion
The fundamental principle to remember is that you cannot directly convert milliliters to grams without knowing the density of the substance. 60 ml of water will have a vastly different mass than 60 ml of mercury, for instance. Water has a density close to 1 g/ml, while mercury is much denser.
Let's break down why:
- Mass: This refers to the amount of matter in an object. It's measured in grams, kilograms, etc.
- Volume: This refers to the amount of space an object occupies. It's measured in milliliters, liters, cubic centimeters, etc.
- Density: This is the link between mass and volume. It tells us how much mass is packed into a given volume. The formula for density is:
Density = Mass / Volume
Therefore, to find the mass (in grams), we need to rearrange the formula:
Mass = Density × Volume
Calculating Mass from Volume and Density: Examples
Let's apply this to a few common substances:
Example 1: Water
Water has a density of approximately 1 g/ml at 4°C (39°F). Therefore, to find the mass of 60 ml of water:
Mass = Density × Volume = 1 g/ml × 60 ml = 60 grams
So, 60 ml of water weighs approximately 60 grams. This is a useful approximation, but keep in mind that the density of water can vary slightly with temperature and pressure.
Example 2: Mercury
Mercury is significantly denser than water. Its density is approximately 13.6 g/ml. To find the mass of 60 ml of mercury:
Mass = Density × Volume = 13.6 g/ml × 60 ml = 816 grams
This shows that 60 ml of mercury weighs a substantial 816 grams, highlighting the significant difference in mass between substances with different densities.
Example 3: Oil
The density of oil varies depending on the type of oil. Let's assume a density of 0.9 g/ml for a particular type of vegetable oil. Then, the mass of 60 ml of this oil would be:
Mass = Density × Volume = 0.9 g/ml × 60 ml = 54 grams
Therefore, 60 ml of this specific type of oil would weigh approximately 54 grams.
The Importance of Accurate Density Values
The accuracy of your mass calculation depends entirely on the accuracy of the density value you use. For many substances, you can find density values in scientific handbooks, online databases, or chemistry textbooks. However, the density of a substance can be affected by several factors, including:
- Temperature: Density generally decreases as temperature increases (with some exceptions).
- Pressure: Higher pressure usually leads to higher density.
- Composition: The precise composition of a substance (e.g., the type of oil) significantly influences its density.
- Phase: The phase of the substance (solid, liquid, or gas) dramatically affects its density.
Therefore, it's crucial to use the most accurate and relevant density value for the specific substance and conditions you are working with.
Beyond Simple Conversions: Applications in Various Fields
Understanding the relationship between volume and mass (and hence, density) is fundamental to various scientific and everyday applications. This knowledge is crucial in:
- Chemistry: Stoichiometry calculations, solution preparation, and determining the concentration of solutions all rely on accurate density measurements.
- Physics: Calculating forces, pressure, and buoyancy depends on knowing the mass and volume of objects.
- Engineering: Design and construction require accurate material property information, including density, to ensure structural integrity.
- Medicine: Dosage calculations for medications often involve considerations of density and volume.
- Cooking: While not as precise as scientific applications, understanding density helps in understanding the behavior of ingredients during cooking. For example, knowing the density of different oils can help you understand how they interact with other ingredients.
Practical Tips for Determining Density
If you need to determine the density of a substance yourself, you can use the following methods:
- Using a graduated cylinder and a balance: Measure the volume of the substance using a graduated cylinder and the mass using a balance. Then, divide the mass by the volume to calculate the density.
- Using a pycnometer: A pycnometer is a specialized device specifically designed for accurate density measurement.
- Using a hydrometer: A hydrometer measures the specific gravity of a liquid, which can be used to calculate its density if the density of water is known.
Conclusion: Context is Key
The question "How many grams are in 60 ml?" doesn't have a single answer. The mass of 60 ml of a substance directly depends on its density. Understanding the concept of density and its relationship to mass and volume is crucial for various scientific, engineering, and everyday applications. Always remember to consider the specific substance and conditions when making such conversions, and utilize accurate density values for the most reliable results. Remember to always double-check your measurements and calculations to ensure accuracy. Precise measurements are critical for ensuring the correct results in any experiment or calculation. By understanding the fundamental principles outlined above, you can confidently tackle similar conversion problems in the future.
Latest Posts
Latest Posts
-
What Is 20 Percent Of 800 000
Mar 17, 2025
-
Words That Start With Y In Science
Mar 17, 2025
-
Prevent An Expressway Emergency By Merging Without
Mar 17, 2025
-
How Many Grams Of Sugar In A Pound
Mar 17, 2025
-
7am To 11am Is How Many Hours
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Are In 60 Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
