How Many Sig Figs Are In 0.020
Arias News
May 12, 2025 · 4 min read
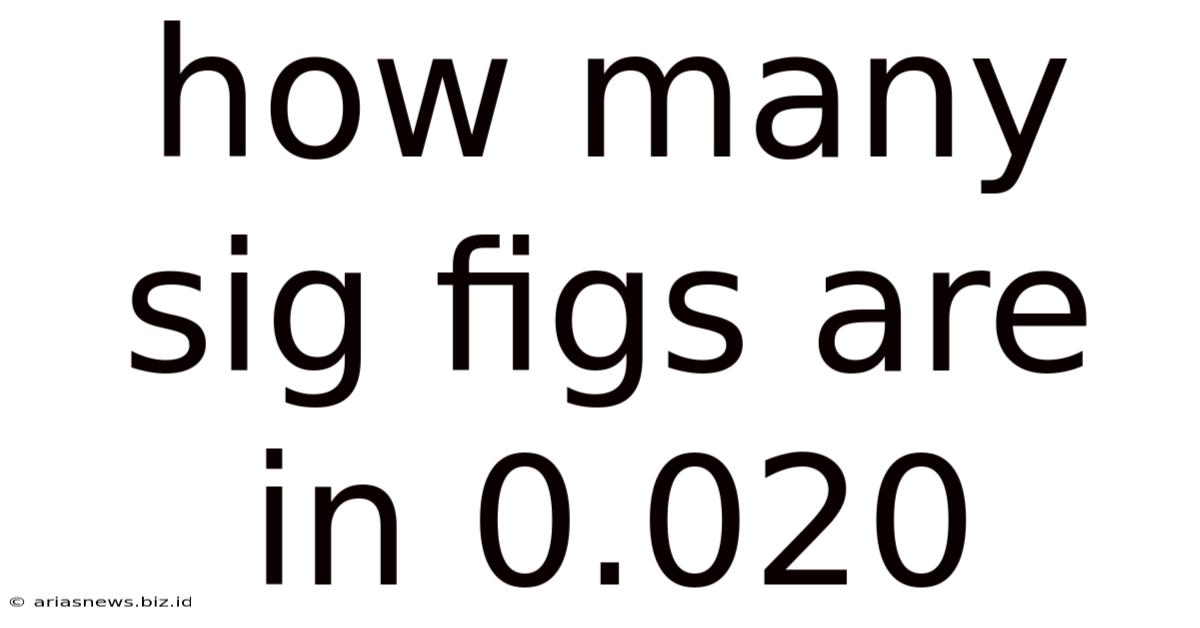
Table of Contents
How Many Significant Figures Are in 0.020? A Deep Dive into Significant Figures
Determining the number of significant figures (sig figs) in a number is crucial for accurate scientific calculations and reporting. While seemingly straightforward, the rules can be confusing, especially with numbers containing leading zeros like 0.020. This article provides a comprehensive guide to understanding significant figures, focusing specifically on the example of 0.020 and exploring the broader implications of sig fig rules in scientific work.
Understanding Significant Figures
Significant figures represent the digits in a number that carry meaning contributing to its precision. They indicate the level of accuracy of a measurement or calculation. The more significant figures a number has, the more precise it is considered. Understanding significant figures is paramount to avoid propagating errors in scientific calculations and ensuring the reliable communication of results.
Rules for Determining Significant Figures
Several rules govern the determination of significant figures:
-
Non-zero digits are always significant. The digits 1, 2, 3, 4, 5, 6, 7, 8, and 9 are always significant regardless of their position in the number.
-
Zeros between non-zero digits are always significant. For example, in the number 1005, the zero is significant.
-
Leading zeros are never significant. These are zeros that precede all non-zero digits. They serve only to locate the decimal point. In 0.0025, the zeros before the 2 are not significant.
-
Trailing zeros in a number containing a decimal point are always significant. In the number 2.00, both trailing zeros are significant.
-
Trailing zeros in a number without a decimal point are ambiguous and may or may not be significant. This ambiguity highlights the importance of scientific notation in conveying precision. For example, 200 could have one, two, or three significant figures depending on the context. To avoid ambiguity, scientific notation should be used.
Analyzing 0.020: A Case Study
Now, let's apply these rules to the number 0.020.
-
Leading zeros: The two zeros to the left of the 2 are leading zeros. According to the rules, these are not significant.
-
Non-zero digit: The digit 2 is a non-zero digit and is therefore significant.
-
Trailing zeros after the decimal point: The zero after the 2 is a trailing zero that follows the decimal point. This zero is therefore significant.
Consequently, the number 0.020 has two significant figures. The leading zeros only serve to position the decimal point and don't contribute to the precision of the measurement.
The Importance of Scientific Notation
Scientific notation provides a clear and unambiguous way to express numbers, especially those with many leading or trailing zeros. It removes the ambiguity associated with trailing zeros in numbers without a decimal point. Scientific notation expresses a number as a value between 1 and 10 multiplied by a power of 10.
For example, the number 0.020 can be expressed in scientific notation as 2.0 x 10<sup>-2</sup>. This notation clearly shows that there are two significant figures. Similarly, a number like 2000 could be written as 2.0 x 10<sup>3</sup> (two significant figures), 2.00 x 10<sup>3</sup> (three significant figures), or 2.000 x 10<sup>3</sup> (four significant figures), eliminating any ambiguity.
Significant Figures in Calculations
The rules for significant figures extend to calculations. When performing calculations involving numbers with different numbers of significant figures, the final answer must be rounded to reflect the least precise measurement.
Addition and Subtraction
In addition and subtraction, the result should have the same number of decimal places as the measurement with the fewest decimal places.
Multiplication and Division
In multiplication and division, the result should have the same number of significant figures as the measurement with the fewest significant figures.
Real-World Applications and Implications
Understanding and applying significant figure rules is crucial in various scientific disciplines. Incorrect handling of significant figures can lead to substantial errors in experimental results, engineering designs, and scientific modeling. For instance, in chemistry, the precision of measurements directly impacts the accuracy of chemical reactions and analyses. In physics, the accuracy of calculations influences the design of structures and equipment.
Avoiding Common Mistakes
A common mistake is neglecting leading zeros or incorrectly interpreting trailing zeros. Always carefully analyze the position of the zeros within the number before determining the number of significant figures. Scientific notation is a valuable tool in avoiding these errors.
Improving Scientific Communication
Consistent and correct use of significant figures enhances the clarity and reliability of scientific communication. It demonstrates attention to detail and rigorous methodology, building trust and credibility within the scientific community.
Conclusion
The number 0.020 contains two significant figures. Mastering the rules for determining significant figures is essential for precise scientific work. Remember to account for leading and trailing zeros correctly, utilize scientific notation to eliminate ambiguity, and apply the appropriate rounding rules during calculations. Precision in reporting and calculating significant figures is crucial for producing reliable and meaningful scientific results. Failing to account for significant figures properly can lead to inaccuracies with substantial consequences, especially in areas where precision is paramount. By understanding and consistently applying the principles of significant figures, we enhance the accuracy, reliability, and communicative power of scientific work.
Latest Posts
Latest Posts
-
What Does Go To The Mattresses Mean
May 12, 2025
-
Caffeine In Crystal Light Peach Mango Green Tea
May 12, 2025
-
Can You Fold A Circle In Halves 15 Different Ways
May 12, 2025
-
How Fast Can A 85cc Dirt Bike Go
May 12, 2025
-
Yond Cassius Has A Lean And Hungry Look
May 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Sig Figs Are In 0.020 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.