Is A Chocolate Chip Cookie A Homogeneous Mixture
Arias News
Mar 18, 2025 · 5 min read
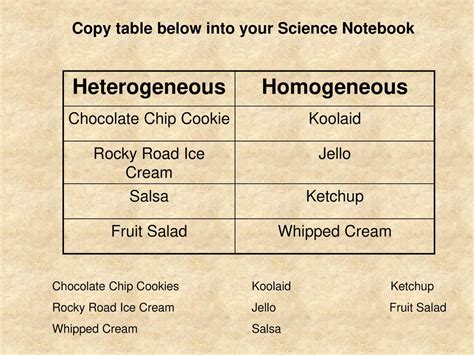
Table of Contents
Is a Chocolate Chip Cookie a Homogeneous Mixture? A Deep Dive into Mixtures and Matter
The seemingly simple question, "Is a chocolate chip cookie a homogeneous mixture?" opens a fascinating exploration into the world of chemistry, specifically the classification of matter. While the answer might seem obvious at first glance, a closer look reveals a more nuanced understanding of mixtures and their properties. This article will delve into the definition of homogeneous mixtures, explore the characteristics of chocolate chip cookies, and ultimately determine the correct classification of this beloved treat.
Understanding Homogeneous Mixtures
Before we dissect a chocolate chip cookie, let's establish a firm grasp on the concept of a homogeneous mixture. In chemistry, a mixture is a substance composed of two or more components that are not chemically bonded. Crucially, these components retain their individual chemical properties. Mixtures can be further categorized into two types: homogeneous and heterogeneous.
A homogeneous mixture is one where the components are uniformly distributed throughout the mixture. This means that at the macroscopic level (what you can see with the naked eye or a standard magnifying glass), the mixture appears to be one single substance. No matter which sample you take from the mixture, its composition will be identical. Think of saltwater: the salt is completely dissolved, and you can't visually distinguish the salt from the water. Other examples include air (a mixture of gases), sugar dissolved in water, and many alloys (like brass).
The Heterogeneous Nature of Chocolate Chip Cookies
Now, let's turn our attention to the star of our investigation: the chocolate chip cookie. A quick visual inspection reveals the answer: a chocolate chip cookie is not a homogeneous mixture. The distinct chocolate chips are clearly visible, embedded within the cookie dough. This immediately disqualifies it from the homogeneous category. Unlike saltwater where the salt is uniformly dispersed, the chocolate chips are concentrated in certain areas, creating a non-uniform distribution.
Macroscopic vs. Microscopic Examination
While the macroscopic observation is sufficient to classify the cookie as heterogeneous, let's take a closer look at a microscopic level. Even if we were to zoom in significantly, the boundaries between the chocolate chips and the cookie dough would remain distinct. The components are not uniformly mixed at a molecular level either. The chocolate chips retain their individual structure and composition, separate from the flour, sugar, butter, and other ingredients in the dough. This reinforces the heterogeneous nature of the cookie.
Exploring the Components of a Chocolate Chip Cookie
To further solidify our understanding, let's break down the individual components of a chocolate chip cookie and analyze their interaction:
- Flour: A complex mixture itself, but in the context of the cookie, it acts as a single component providing structure.
- Sugar: Granulated sugar dissolves partially in the dough, but some crystals remain intact.
- Butter: Provides fat and contributes to the texture. It emulsifies with other ingredients, but doesn't uniformly distribute at a microscopic level.
- Eggs: Act as a binder, holding the ingredients together.
- Chocolate Chips: These are the most readily identifiable separate phase. They retain their individual identity and form throughout the baking process.
Each of these components retains its unique physical and chemical properties within the cookie. They do not combine to form a new substance, a characteristic of chemical reactions rather than mixtures. The interaction between these components is primarily physical, leading to a complex, multi-phased structure.
Distinguishing Homogeneous and Heterogeneous Mixtures: Key Differences
To reinforce the understanding of the differences between homogeneous and heterogeneous mixtures, let's highlight the key distinctions:
| Feature | Homogeneous Mixture | Heterogeneous Mixture |
|---|---|---|
| Uniformity | Uniform composition throughout | Non-uniform composition |
| Visible Components | Components are not visible | Components are visible |
| Separation | Components cannot be easily separated by physical means | Components can be easily separated by physical means (e.g., filtration, decantation) |
| Examples | Saltwater, air, sugar in water | Chocolate chip cookie, sand and water, salad |
The Role of Baking in the Cookie's Heterogeneity
The baking process doesn't alter the fundamental heterogeneous nature of the cookie. While the ingredients undergo physical and chemical changes during baking (such as the Maillard reaction, which contributes to browning), these changes do not result in a uniform distribution of components. The chocolate chips remain distinct entities, even after being baked and incorporated into the cookie dough matrix.
Beyond Chocolate Chip Cookies: Applying the Concept
Understanding the difference between homogeneous and heterogeneous mixtures extends far beyond the realm of baking. This fundamental concept is crucial in various scientific fields, including:
- Materials Science: Analyzing the properties of alloys and composites.
- Environmental Science: Studying the composition of air and water.
- Food Science: Understanding the texture and properties of various food products.
- Pharmacology: Formulating medications and ensuring uniform drug delivery.
The ability to accurately classify mixtures is essential for understanding their behavior and predicting their properties.
Conclusion: The Chocolate Chip Cookie Verdict
In conclusion, the evidence overwhelmingly supports the classification of a chocolate chip cookie as a heterogeneous mixture. The readily visible and distinctly separate chocolate chips within the cookie dough, along with the non-uniform distribution of components at both macroscopic and microscopic levels, definitively places it in the heterogeneous category. While delicious and complex in its flavor profile, its structure firmly aligns with the definition of a heterogeneous mixture. This example underscores the importance of careful observation and understanding of the fundamental principles of chemistry in categorizing matter. The next time you enjoy a chocolate chip cookie, remember the scientific marvel of its heterogeneous composition!
Latest Posts
Latest Posts
-
How Many Cups In Pound Of Pasta
Mar 18, 2025
-
How Many Ounces In A Handle Of Vodka
Mar 18, 2025
-
How Many Cups Of Water Is 20 Oz
Mar 18, 2025
-
Price Per Lb To Price Per Kg
Mar 18, 2025
-
How Do You Say Emma In Spanish
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is A Chocolate Chip Cookie A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
