What Color Would Litmus Paper Red Turn In Cleaning Spray
Arias News
Mar 21, 2025 · 6 min read
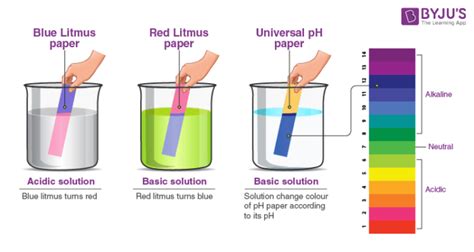
Table of Contents
What Color Would Litmus Paper Turn Red in Cleaning Spray? Understanding pH and Cleaning Solutions
Cleaning sprays are ubiquitous in our homes, promising sparkling surfaces and a fresh scent. But have you ever wondered about the chemical composition of these convenient cleaners? Understanding their pH level, using a simple tool like litmus paper, can offer valuable insights into their effectiveness and potential safety considerations. This article delves deep into the fascinating world of cleaning sprays, pH levels, and the color changes observed with litmus paper, specifically focusing on what happens when red litmus paper is exposed to various cleaning solutions.
Understanding pH and Litmus Paper
Before we dive into the specifics of cleaning sprays, let's establish a basic understanding of pH and how litmus paper works. The pH scale measures the acidity or alkalinity of a substance. It ranges from 0 to 14, with 7 being neutral. A pH below 7 indicates acidity (like lemon juice), while a pH above 7 indicates alkalinity (like baking soda).
Litmus paper is a simple indicator used to test pH. It's typically made from a mixture of dyes extracted from lichens. There are two types:
- Red litmus paper: Turns blue in the presence of an alkaline substance (pH > 7).
- Blue litmus paper: Turns red in the presence of an acidic substance (pH < 7).
Common Ingredients in Cleaning Sprays and Their pH Levels
Cleaning sprays contain a variety of ingredients, each contributing to their cleaning power and specific properties. The pH of a cleaning spray is determined by the combination of these ingredients. Some common ingredients and their typical pH ranges include:
- Water: Neutral (pH 7)
- Surfactants: These reduce surface tension, allowing the cleaner to penetrate and lift dirt and grime. Their pH can vary depending on the specific surfactant used. Some are acidic, others are alkaline, and some are neutral.
- Acids: Many cleaning sprays contain acids like citric acid (found in citrus fruits) or lactic acid (found in milk) to help dissolve mineral deposits and grime. These will generally have a pH below 7.
- Alkalis: Some cleaning sprays, particularly those designed for heavy-duty cleaning or grease removal, contain alkalis like ammonia or sodium hydroxide. These will have a pH above 7.
- Bleaches: Bleach (sodium hypochlorite) is a strong oxidizing agent used to disinfect and whiten. It's strongly alkaline, with a pH typically above 11.
- Fragrances and Preservatives: These components don't significantly affect the overall pH of the cleaning solution but contribute to the scent and shelf life.
What Happens When Red Litmus Paper is Exposed to Cleaning Spray?
The color change observed when red litmus paper is dipped into a cleaning spray depends entirely on the spray's pH.
-
If the cleaning spray is acidic (pH < 7): The red litmus paper will remain red. No color change will occur because the solution is already acidic, and red litmus paper only changes color in the presence of an alkaline solution. Examples of cleaning sprays likely to show this reaction include those containing citric acid or vinegar.
-
If the cleaning spray is neutral (pH 7): Again, the red litmus paper will remain red. A neutral pH won't cause a color change in red litmus paper. This is unlikely for a commercial cleaning spray, but some homemade cleaning solutions might be close to neutral.
-
If the cleaning spray is alkaline (pH > 7): The red litmus paper will turn blue. This indicates the presence of an alkaline substance. Cleaning sprays containing ammonia, bleach, or other alkalis will cause this color change. The intensity of the blue color can give an indication of how strongly alkaline the solution is; a deeper blue suggests a higher pH.
Interpreting the Results: What the Color Change Means
The color change, or lack thereof, provides valuable information about the cleaning spray's properties:
-
Red remains red: The cleaning spray is either acidic or neutral. Acidic cleaners are generally effective at removing mineral deposits and soap scum.
-
Red turns blue: The cleaning spray is alkaline. Alkaline cleaners are usually more effective at cutting through grease and grime. However, they can be harsher on certain surfaces.
Safety Precautions When Testing Cleaning Sprays
Always exercise caution when working with cleaning sprays and chemicals.
- Wear appropriate protective gear: Gloves and eye protection are recommended when handling cleaning solutions, especially those with strong alkaline properties.
- Work in a well-ventilated area: Many cleaning sprays contain volatile organic compounds (VOCs) that can be harmful if inhaled in high concentrations.
- Follow the manufacturer's instructions: Always refer to the product label for safety information and usage instructions.
- Keep out of reach of children and pets: Cleaning sprays should be stored securely to prevent accidental ingestion or exposure.
- Dispose of properly: Follow local regulations for the disposal of cleaning product containers.
Different Types of Cleaning Sprays and Their Likely pH
To further illustrate the relationship between cleaning spray type and litmus paper reaction, let's look at some specific examples:
-
Glass cleaner: Many glass cleaners are slightly acidic to help dissolve mineral deposits and leave streak-free surfaces. Red litmus paper would likely remain red.
-
All-purpose cleaner: These cleaners vary greatly in their formulation. Some are acidic, others are alkaline, and some are closer to neutral. The litmus paper test would provide a clear indication of the pH.
-
Bathroom cleaner: Bathroom cleaners often contain acids to remove soap scum and mineral deposits, or alkalis to tackle mildew and mold. The result on red litmus paper would depend on the specific formulation.
-
Oven cleaner: Oven cleaners are typically strongly alkaline to dissolve baked-on grease and food. Red litmus paper would almost certainly turn blue.
-
Disinfectant spray: Disinfectants can be acidic or alkaline, depending on their active ingredients. The litmus paper test would indicate the pH.
Beyond Litmus Paper: Other Methods for Determining pH
While litmus paper provides a quick and easy way to determine if a cleaning spray is acidic or alkaline, more precise pH measurements can be obtained using a pH meter or pH indicator solutions. These methods offer a numerical pH value, providing a more detailed understanding of the solution's acidity or alkalinity.
Conclusion: Litmus Paper as a Useful Tool
Litmus paper offers a simple yet effective way to gain insight into the pH of cleaning sprays. Understanding the pH is crucial for selecting the appropriate cleaner for a specific task, ensuring the safety of use, and understanding the potential impact on different surfaces. While the color change of red litmus paper provides a general indication of acidity or alkalinity, more precise methods can be employed for a more detailed analysis. Always prioritize safety when handling cleaning sprays and chemicals. By understanding the basic principles of pH and employing simple tests like litmus paper, we can make informed choices about cleaning products and ensure a safer and more effective cleaning process. Remember to always read and follow the manufacturer's instructions on the cleaning spray label.
Latest Posts
Latest Posts
-
How Long Is 48 Weeks In Months
Mar 28, 2025
-
How Many Square Miles Is 5000 Acres
Mar 28, 2025
-
How Many Blades Of Grass On Earth
Mar 28, 2025
-
Is It Illegal To Dumpster Dive In Kansas
Mar 28, 2025
-
Which Sentence Is The Best Example Of Alliteration
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Color Would Litmus Paper Red Turn In Cleaning Spray . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
