What Element Has 5 Energy Levels And 3 Valence Electrons
Arias News
Mar 18, 2025 · 5 min read
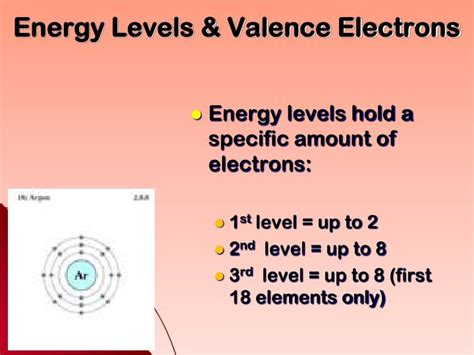
Table of Contents
What Element Has 5 Energy Levels and 3 Valence Electrons? Unraveling the Mystery of Aluminum
The question, "What element has 5 energy levels and 3 valence electrons?" might seem like a niche query, but it's a great example of how fundamental principles in chemistry can lead us to a specific element. Understanding electron configuration, energy levels, and valence electrons is key to unlocking the answer and appreciating the periodic table's organization. This article delves deep into this question, explaining the concepts involved and ultimately revealing the element in question. We'll also explore its properties, applications, and importance in various fields.
Understanding Electron Configuration and Energy Levels
Before we dive into identifying the element, let's clarify the core concepts:
Electron Configuration: This describes the arrangement of electrons in an atom's energy levels and sublevels. It's like an atom's address, specifying where each electron resides. We use a notation system involving numbers and letters (e.g., 1s², 2s², 2p⁶, etc.) to represent this arrangement.
Energy Levels (Principal Quantum Numbers): These are distinct regions surrounding the nucleus where electrons are likely to be found. They're denoted by the principal quantum number, n, which can be any positive integer (1, 2, 3, etc.). The higher the n value, the further the energy level is from the nucleus and the higher the energy of the electrons within it.
Sublevels: Within each energy level (except for n = 1), electrons occupy sublevels, which are further subdivisions of energy. These are labeled as s, p, d, and f. Each sublevel can hold a specific number of electrons: s (2 electrons), p (6 electrons), d (10 electrons), and f (14 electrons).
Valence Electrons: These are the electrons in the outermost energy level of an atom. They are the electrons most involved in chemical bonding and determine an element's chemical properties and reactivity.
Deciphering the Clues: 5 Energy Levels and 3 Valence Electrons
The problem states that our mystery element possesses five energy levels (n = 1 to 5) and three valence electrons. This significantly narrows down the possibilities. The presence of five energy levels means the element is located in the fifth period (row) of the periodic table. The three valence electrons indicate that it resides in Group 13 (also known as Group IIIA), which is characterized by elements with three electrons in their outermost shell.
Let's build a hypothetical electron configuration based on these clues:
The first three energy levels would be completely filled:
- Level 1: 2 electrons (1s²)
- Level 2: 8 electrons (2s²2p⁶)
- Level 3: 8 electrons (3s²3p⁶)
- Level 4: 18 electrons (4s²3d¹⁰4p⁶)
Now, let's move to energy level 5. Since the element has 3 valence electrons, we know there are 3 electrons in this level:
- Level 5: 3 electrons (5s²5p¹)
This gives us a partial electron configuration of 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²5p¹.
However, the most stable configuration for this element is slightly different due to the filling order of orbitals and exceptions in the periodic table. The 4s orbital fills before the 3d, and the 5s orbital similarly fills before the 4d and 5p.
Putting all this together, the element with 5 energy levels and 3 valence electrons is Aluminum (Al).
Aluminum: Properties, Applications, and Importance
Now that we've identified the element, let's explore its characteristics and significance:
Properties of Aluminum:
- Lightweight: Aluminum is significantly lighter than most other metals, making it ideal for various applications where weight is a concern.
- High Strength-to-Weight Ratio: Despite its lightness, aluminum possesses considerable strength, contributing to its versatility.
- Excellent Conductivity: Aluminum is a good conductor of electricity and heat, making it suitable for electrical wiring, cookware, and heat sinks.
- Corrosion Resistance: Aluminum forms a protective oxide layer on its surface, which protects it from further corrosion. This makes it durable and resistant to rusting.
- Malleability and Ductility: It can be easily shaped and drawn into wires, enabling its use in a wide array of products.
- Abundant and Recyclable: Aluminum is a relatively abundant element and can be recycled repeatedly without significant loss of properties.
Applications of Aluminum:
The versatility of aluminum has led to its extensive use in diverse sectors:
- Transportation: It is widely used in automobiles, aircraft, trains, and ships, due to its lightweight and strength properties.
- Packaging: Aluminum foil and cans are ubiquitous in the food and beverage industry.
- Construction: Aluminum is employed in building materials, such as window frames, siding, and roofing.
- Electrical Engineering: Its conductivity makes it crucial for electrical wiring, power transmission lines, and circuit boards.
- Consumer Goods: Aluminum features in various consumer products, including cookware, appliances, and sporting goods.
- Aerospace: Due to its high strength-to-weight ratio, it's vital in aircraft and spacecraft construction.
Environmental Importance and Recycling:
The recyclability of aluminum is environmentally significant. Recycling aluminum requires significantly less energy than producing it from raw materials, thus reducing carbon emissions and conserving natural resources. Its widespread recycling contributes to sustainable practices and minimizes environmental impact.
Aluminum Alloys:
Aluminum is rarely used in its pure form. It’s frequently alloyed with other elements to enhance its specific properties. Different alloying elements change the mechanical and physical characteristics of aluminum, making it suitable for various applications. For instance, adding copper increases its strength, while adding magnesium improves its weldability.
The Chemistry Behind Aluminum's Reactivity:
Aluminum's three valence electrons are readily available for chemical bonding, explaining its reactivity. These electrons are easily lost, forming Al³⁺ ions which are stable and readily participate in ionic compounds.
Conclusion: A Journey Through the Periodic Table
By carefully analyzing the clues provided – five energy levels and three valence electrons – we successfully identified the element as aluminum. This journey highlighted the importance of understanding electron configuration, energy levels, and valence electrons in characterizing elements and predicting their properties. Aluminum's widespread applications, combined with its environmental benefits, underline its importance in modern society and the continuing relevance of understanding fundamental chemistry principles. This example showcases how seemingly specific questions in chemistry can lead to deeper appreciation for the organization and properties of the elements within the periodic table.
Latest Posts
Latest Posts
-
How To Say Passion Fruit In Spanish
Mar 18, 2025
-
How Many Hours Are In 180 Minutes
Mar 18, 2025
-
How Many Acers Are In A Mile
Mar 18, 2025
-
Are Matthew Garber And Victor Garber Related
Mar 18, 2025
-
What Is Half Of 2 3 4
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Element Has 5 Energy Levels And 3 Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
