Which Elements Will Most Likley Form Anions
Arias News
Apr 01, 2025 · 5 min read
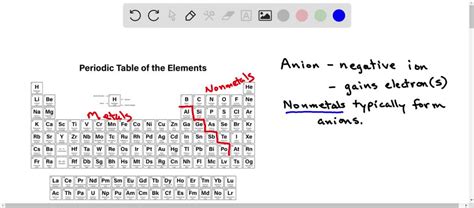
Table of Contents
Which Elements Will Most Likely Form Anions?
Understanding which elements readily form anions is fundamental to chemistry. Anions, negatively charged ions, are crucial in various chemical processes, from forming ionic compounds to participating in biological reactions. This article delves deep into the periodic trends that dictate anion formation, exploring the key factors and providing examples to solidify your understanding.
Electronegativity: The Driving Force Behind Anion Formation
The most significant factor determining an element's propensity to form anions is its electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity have a strong pull on electrons, making them more likely to gain electrons and form negatively charged ions (anions).
Understanding the Periodic Trend
Electronegativity generally increases as you move across a period (from left to right) on the periodic table and decreases as you move down a group (from top to bottom). This trend is directly related to the effective nuclear charge and atomic radius.
-
Across a Period: As you move across a period, the number of protons in the nucleus increases, resulting in a stronger positive charge attracting the electrons more effectively. The atomic radius generally decreases across a period, bringing the valence electrons closer to the nucleus, further enhancing the attraction.
-
Down a Group: As you move down a group, the number of electron shells increases, placing the valence electrons farther from the nucleus. This increased distance reduces the effective nuclear charge experienced by the valence electrons, weakening the attraction and decreasing electronegativity.
Nonmetals: The Anion Kings
Nonmetals, located on the right side of the periodic table, are the primary elements that form anions. Their high electronegativity makes them eager to accept electrons to achieve a stable electron configuration, often resembling a noble gas (octet rule).
Halogens: The Masters of Anion Formation
The halogens (Group 17: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)) are exceptionally adept at forming anions. They only need to gain one electron to achieve a full octet, resulting in a -1 charge (e.g., F⁻, Cl⁻, Br⁻, I⁻). Their high electronegativity and the relatively small energy change required to gain one electron make them highly reactive and prone to anion formation.
Chalcogens: Often Forming -2 Anions
The chalcogens (Group 16: oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po)) readily form anions with a -2 charge. They need to gain two electrons to achieve a noble gas configuration (e.g., O²⁻, S²⁻, Se²⁻). Oxygen, in particular, is highly electronegative and forms anions in countless compounds.
Pnictogens: -3 Anions and Beyond
The pnictogens (Group 15: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi)) can form anions, though they are less common than halogens or chalcogens. They typically require three electrons to reach a stable octet, resulting in a -3 charge (e.g., N³⁻, P³⁻). However, the tendency to form anions decreases as you move down the group, with bismuth less likely to form a -3 anion.
Other Elements Forming Anions: Beyond the Nonmetals
While nonmetals are the most likely candidates for anion formation, some other elements under specific circumstances can also form anions.
Transition Metals: Anions in Specific Complexes
Some transition metals, while typically forming cations, can form complex anions under specific conditions. These complexes usually involve coordination with ligands (molecules or ions that donate electrons), stabilizing the negative charge. For instance, certain transition metal complexes with cyanide (CN⁻) ligands can form anionic complexes.
Metalloids: A Gray Area
Metalloids (boron, silicon, germanium, arsenic, antimony, tellurium, and polonium) exhibit properties intermediate between metals and nonmetals. While less common than in nonmetals, they can form anions under certain circumstances, often with highly electronegative elements.
Factors Affecting Anion Formation Beyond Electronegativity
While electronegativity is the dominant factor, other factors can influence an element's ability to form anions:
-
Ionization Energy: The energy required to remove an electron from an atom. Lower ionization energies generally favor anion formation since it's easier to add electrons.
-
Electron Affinity: The change in energy when an electron is added to a neutral atom. A high electron affinity indicates a strong attraction for an additional electron, favoring anion formation.
-
Lattice Energy: In ionic compounds, the energy released when ions come together to form a crystal lattice. Higher lattice energy stabilizes the ionic compound, making anion formation more favorable.
-
Size and Charge: Larger ions can better accommodate additional electrons, while a high negative charge requires a large ion to stabilize.
Examples of Anion Formation in Common Compounds
Many everyday compounds involve anions:
-
Sodium Chloride (NaCl): Sodium (Na) forms a +1 cation, while chlorine (Cl) forms a -1 anion (chloride ion).
-
Magnesium Oxide (MgO): Magnesium (Mg) forms a +2 cation, while oxygen (O) forms a -2 anion (oxide ion).
-
Calcium Phosphate (Ca₃(PO₄)₂): Calcium (Ca) forms a +2 cation, while phosphate (PO₄) forms a -3 anion.
Conclusion: Predicting Anion Formation
Predicting which elements will most likely form anions primarily relies on understanding periodic trends in electronegativity. Nonmetals, particularly halogens and chalcogens, are the most prone to anion formation due to their high electronegativity and the relatively small energy required to achieve a stable electron configuration. While other factors influence anion formation, electronegativity remains the key determinant. Mastering this concept is crucial for understanding chemical bonding, reactivity, and the properties of countless compounds. By applying this knowledge, you can confidently predict the likelihood of anion formation for various elements and understand the fundamental principles governing their behavior in chemical reactions.
Latest Posts
Latest Posts
-
Where Do You Put The Date On A Letter
Apr 02, 2025
-
How Much Money Is A Pound Of Pennies
Apr 02, 2025
-
Born In 78 How Old Am I
Apr 02, 2025
-
Dog Breed That Starts With An X
Apr 02, 2025
-
6 And 1 Half Dozen The Other
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Elements Will Most Likley Form Anions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
