1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
Arias News
May 11, 2025 · 6 min read
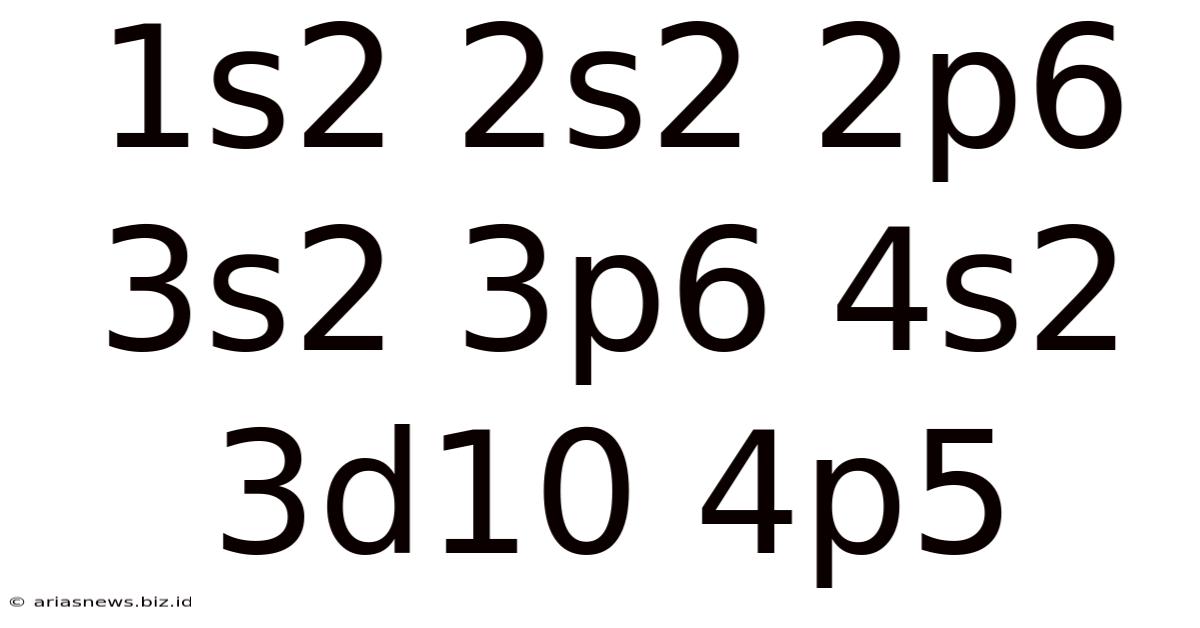
Table of Contents
Unveiling the Mystery: A Deep Dive into the Electron Configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
The seemingly simple string of numbers and letters, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵, represents far more than just a collection of symbols. It's a concise yet powerful description of the electronic structure of an atom, revealing fundamental properties that dictate its chemical behavior and place in the periodic table. This electron configuration corresponds to the element bromine (Br), a halogen known for its reddish-brown color and distinctive reactivity. Let's embark on a detailed exploration of this configuration, delving into the underlying principles and implications.
Understanding Electron Configurations: The Building Blocks of Atoms
Before diving into the specifics of bromine's electron configuration, it's essential to grasp the foundational concepts. Electron configuration describes how electrons are distributed among various energy levels and orbitals within an atom. These orbitals are regions of space where there's a high probability of finding an electron. The configuration follows specific rules dictated by quantum mechanics:
The Aufbau Principle: Filling Orbitals in Order of Increasing Energy
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy. This means electrons occupy the lowest energy levels first before moving to higher energy levels. This filling order is not always straightforward, as the energy levels of orbitals can sometimes overlap.
Hund's Rule: Maximizing Unpaired Electrons
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before pairing up. This minimizes electron-electron repulsion and leads to greater stability.
Pauli Exclusion Principle: One Electron per Spin State
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This essentially means that each orbital can hold a maximum of two electrons, each with opposite spins (spin up and spin down).
Deconstructing 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵: A Step-by-Step Analysis
Now, let's break down the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵ piece by piece:
-
1s²: The '1' represents the principal quantum number (n), indicating the energy level. 's' denotes the subshell, which is a spherical orbital. The superscript '2' signifies that there are two electrons in this 1s orbital, one with spin up and one with spin down.
-
2s²: Similarly, '2' represents the second energy level, and 's' represents the s subshell. Again, there are two electrons in this orbital.
-
2p⁶: '2' is the second energy level, and 'p' represents the p subshell, which consists of three dumbbell-shaped orbitals (px, py, pz). The superscript '6' indicates that all three orbitals are filled with two electrons each (a total of six electrons).
-
3s² and 3p⁶: These follow the same logic as the previous levels, representing the completely filled third energy level with two electrons in the 3s subshell and six electrons in the 3p subshell.
-
4s²: This indicates two electrons in the 4s subshell, which is in the fourth energy level. Note that even though the 4s subshell fills before the 3d subshell in accordance with the Aufbau principle, and is slightly lower in energy.
-
3d¹⁰: Here we find the 3d subshell within the third energy level. The 'd' subshell has five orbitals, each capable of holding two electrons. The superscript '10' means all five orbitals are completely filled with ten electrons.
-
4p⁵: Finally, we reach the 4p subshell in the fourth energy level. This subshell has three orbitals, and the superscript '5' indicates that five electrons occupy these orbitals. According to Hund's rule, these five electrons will each occupy a separate orbital before pairing up. This leaves one orbital unoccupied, contributing to bromine's reactivity.
Implications of Bromine's Electron Configuration: Chemical Behavior and Properties
Bromine's electron configuration directly influences its chemical and physical properties. The presence of seven valence electrons (electrons in the outermost shell, 4s² and 4p⁵) is crucial. This nearly complete outermost shell is the reason why bromine is highly reactive. It readily gains one electron to achieve a stable octet configuration (like the noble gas krypton), forming bromide ions (Br⁻).
Reactivity and Formation of Compounds
Bromine's high reactivity is evident in its tendency to form ionic and covalent compounds. It readily reacts with metals to form ionic bromides (e.g., sodium bromide, NaBr), where it accepts an electron from the metal. It also forms covalent bonds with nonmetals, sharing electrons to complete its octet (e.g., hydrogen bromide, HBr).
Physical Properties and Atomic Radius
The electron configuration also contributes to bromine's physical properties. Its relatively large atomic radius compared to other halogens is due to the increased number of electron shells. Its reddish-brown color is a result of the absorption and emission of light by its electrons. Its liquid state at room temperature is unusual for halogens, and is related to the strength of intermolecular forces.
Comparing Bromine to Other Elements: Periodic Trends
Understanding bromine's electron configuration allows us to compare it to other elements and observe periodic trends.
Comparison with Other Halogens
Bromine belongs to Group 17, the halogens. Comparing its electron configuration to other halogens (fluorine, chlorine, iodine, astatine) reveals a pattern: they all have seven valence electrons, resulting in similar chemical properties, although their reactivity varies due to differences in electronegativity and atomic size.
Ionization Energy and Electronegativity
Bromine's electron configuration impacts its ionization energy (the energy required to remove an electron) and electronegativity (the tendency to attract electrons in a chemical bond). Its high electronegativity is a direct consequence of its almost-complete outer shell, driving it to gain an electron and achieve stability.
Bromine's Role in the World: Applications and Importance
Bromine's unique properties, stemming from its electron configuration, lead to a range of applications:
-
Flame Retardants: Brominated flame retardants are used in various materials to prevent or slow the spread of fire.
-
Water Treatment: Bromine compounds are used in water purification to disinfect and kill harmful bacteria and microorganisms.
-
Agricultural Chemicals: Some bromine compounds are used as pesticides and fungicides.
-
Pharmaceuticals: Bromine is a component in certain pharmaceuticals and medicines.
-
Photography: Historically, bromine compounds were used in photography.
Conclusion: A Deeper Appreciation of Atomic Structure
The seemingly simple electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵ holds the key to understanding bromine's behavior and properties. By dissecting this configuration and understanding the underlying principles of quantum mechanics, we gain a deeper appreciation for the intricate relationship between atomic structure and macroscopic properties. This knowledge is crucial in various fields, from chemistry and materials science to environmental science and medicine. Bromine, with its reactive nature and diverse applications, serves as a compelling example of how the arrangement of electrons within an atom dictates its role in the world around us. Further exploration of periodic trends and related elements expands this understanding even more. This microscopic view, therefore, allows for a broader comprehension of macroscopic reality.
Latest Posts
Latest Posts
-
Does Time Go On The X Axis
May 12, 2025
-
What Is 2 3 Cup Times 3
May 12, 2025
-
How Long Is A Couple Of Hours
May 12, 2025
-
What Instruments Are Used In Blues Music
May 12, 2025
-
How Many Times Can You Get Married In Maine
May 12, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.