How Many Units Is In 1 Ml
Arias News
Mar 16, 2025 · 6 min read
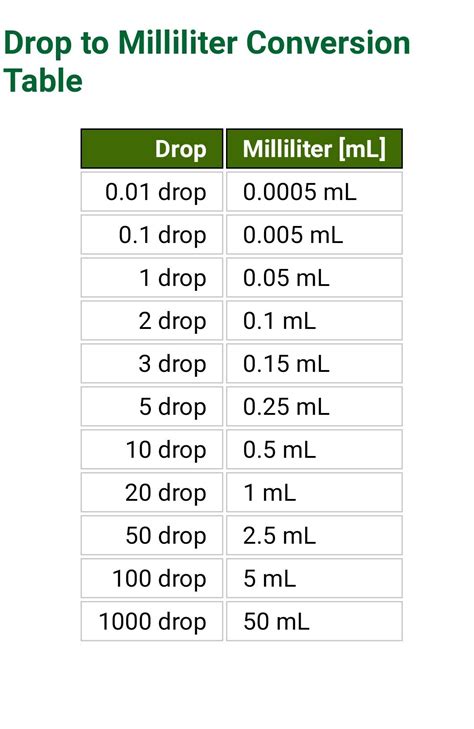
Table of Contents
How Many Units Are in 1 mL? Understanding Volume and Concentration
The question "How many units are in 1 mL?" doesn't have a single, straightforward answer. It's a bit like asking "How many apples are in a basket?" – it depends entirely on the size of the apples (concentration) and the size of the basket (volume). In this case, "units" refers to the quantity of a substance dissolved within the 1 mL volume. This could be anything from medication dosages to chemical concentrations. Therefore, understanding the context is crucial. This article will delve into various scenarios where this question arises and provide methods to calculate the number of "units" in 1 mL.
Understanding the Concept of Concentration
Before we dive into specific examples, let's clarify the concept of concentration. Concentration refers to the amount of a solute (the substance being dissolved) present in a given amount of solvent (the substance doing the dissolving) or solution (the solute and solvent combined). Concentration is typically expressed in various units, including:
- Molarity (M): Moles of solute per liter (L) of solution. This is a very common unit in chemistry.
- Molality (m): Moles of solute per kilogram (kg) of solvent. This is less affected by temperature changes than molarity.
- Normality (N): Equivalents of solute per liter (L) of solution. Used for reactions involving acids and bases.
- Parts per million (ppm) and parts per billion (ppb): Mass units of solute per million or billion mass units of solution. Often used for very dilute solutions.
- Percentage (%): Grams of solute per 100 mL or 100 g of solution. Can be expressed as weight/volume (%) or weight/weight (%).
- Units/mL or Units/L: This is a general term used when the specific unit of the solute is defined, such as IU/mL (International Units per milliliter) for some medications.
Calculating Units in 1 mL: Different Scenarios
Let's explore several scenarios to illustrate how to calculate the number of "units" in 1 mL:
1. Pharmaceutical Preparations: Medications and Injections
Many injectable medications are labeled with units per milliliter (e.g., IU/mL, mg/mL). This directly tells you the number of units in 1 mL. For instance:
- Insulin: A vial of insulin might be labeled as 100 IU/mL. This means there are 100 International Units of insulin in 1 mL of the solution.
- Heparin: Heparin, an anticoagulant, might have a concentration of 5000 IU/mL. In this case, 1 mL contains 5000 International Units of heparin.
If the concentration is given in a different unit, such as mg/mL (milligrams per milliliter), you'll need to know the conversion factor between the desired unit and mg.
2. Chemical Solutions: Molarity and Other Concentrations
In chemistry, concentrations are frequently expressed in molarity (M). To determine the number of moles (a unit of substance amount) in 1 mL, you need to convert the volume to liters and then use the molarity:
-
Example: A solution has a concentration of 0.5 M (0.5 moles/liter). To find the moles in 1 mL (0.001 L):
0.5 moles/L * 0.001 L = 0.0005 moles
Therefore, there are 0.0005 moles in 1 mL of this 0.5 M solution. To express this in other units, you would need the molar mass of the solute.
Similar calculations can be done with other concentration units, such as molality, normality, etc., depending on the given information. You will need to use the appropriate conversion factors.
3. Dilutions and Conversions
Often, you'll need to prepare a solution of a desired concentration by diluting a stock solution. This involves calculating the volume of stock solution needed to achieve the target concentration.
-
Example: You have a 10 M stock solution and you need 100 mL of a 0.1 M solution. You can use the dilution formula:
C1V1 = C2V2
Where: * C1 = concentration of stock solution (10 M) * V1 = volume of stock solution (unknown) * C2 = concentration of diluted solution (0.1 M) * V2 = volume of diluted solution (100 mL = 0.1 L)
Solving for V1:
V1 = (C2V2) / C1 = (0.1 M * 0.1 L) / 10 M = 0.001 L = 1 mL
Therefore, you need 1 mL of the 10 M stock solution to prepare 100 mL of a 0.1 M solution. The number of "units" in this 1 mL would then depend on the nature of the solute.
Important Note: Always remember to use consistent units throughout your calculations (e.g., convert mL to L when working with molarity).
4. Units Specific to a Substance
Some substances have units specific to their activity or effect. For instance:
- Enzyme activity: Enzyme activity is often expressed in units/mL, where the unit describes the amount of enzyme needed to catalyze a specific reaction under defined conditions. The specific definition of the unit depends on the enzyme.
- Antibiotic potency: Potency can be expressed in various units, such as mcg/mL (micrograms per milliliter).
The Importance of Proper Labeling and Units
Precise labeling and the clear indication of units are paramount, particularly in medicine, chemistry, and other scientific fields. Ambiguity can lead to serious errors with potentially hazardous consequences. Always double-check the units and ensure that you understand the context before making any calculations or using the solution.
Practical Applications and Examples
Let's consider a few real-world examples where understanding "units per mL" is crucial:
- Pharmacy: Pharmacists must accurately calculate dosages based on the concentration of medications. Incorrect calculations can have severe health implications.
- Clinical Laboratory: In a clinical laboratory, accurate concentration measurements are essential for diagnosing diseases and monitoring patient health. For example, determining the concentration of glucose or cholesterol in blood samples requires precise units and measurements.
- Environmental Science: In environmental monitoring, accurate measurements of pollutants in water or soil samples are crucial for assessing environmental health and taking appropriate actions. The units used need to be accurately reported and interpreted.
- Food Science: In food science, understanding the concentration of various ingredients is important for quality control and ensuring food safety.
Conclusion: Context is Key
The number of "units" in 1 mL is highly context-dependent. The answer necessitates knowing the concentration of the substance in question and its units of measurement. Whether it's medication dosage, chemical concentration, or enzyme activity, understanding concentration and units is fundamental for accurate calculations and safe practice. Always carefully check labels, use the correct conversion factors, and employ appropriate dilution techniques when working with solutions to avoid mistakes and ensure accuracy. The focus should always be on precise measurements and accurate calculations to prevent errors that can have serious repercussions in various fields. Remember to always consult relevant guidelines and safety protocols when working with chemicals or pharmaceuticals.
Latest Posts
Latest Posts
-
How Many Ounces In A Loaf Of Bread
May 09, 2025
-
How Much Is 2 3 Cup Doubled
May 09, 2025
-
30 Tens 3 Tens 3 Tenths
May 09, 2025
-
What Is The Average Height Of A 4th Grader
May 09, 2025
-
Do Kris And Junior End Up Together
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Units Is In 1 Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.